Gypsum: Difference between revisions
Jump to navigation
Jump to search
No edit summary |
No edit summary |
||
| Line 1: | Line 1: | ||
{{Infobox_Salt | {{Infobox_Salt | ||
|Footnote = | |Footnote = <ref>http://webmineral.com/data/Gypsum.shtml seen on 30.07.2010</ref><ref>http://www.mindat.org/min-1784.html seen on 30.07.2010</ref> | ||
|photo = | |photo = SA101 1.jpeg|300px | ||
|mineralogical_Name = | |mineralogical_Name = Gipsum, Selenite | ||
|chemical_Name = | |chemical_Name = Calcium sulphate dihydrate | ||
|Trivial_Name = | |Trivial_Name = Gypsite, Sulphate of Lime | ||
|chemical_Formula | |chemical_Formula = Ca[SO<sub>4</sub>]•2H<sub>2</sub>O | ||
|Hydratforms = | |Hydratforms = Anhydrite (CaSO<sub>4</sub>)<br>Hemihydrate (CaSO<sub>4</sub>•0,5H<sub>2</sub>O) | ||
|Crystal_Class = | |Crystal_Class = monoclinic | ||
|Crystal_Structure = | |Crystal_Structure = | ||
|Deliqueszenzhumidity = | |Deliqueszenzhumidity = | ||
|Solubility = | |Solubility = | ||
|Density = | |Density = 2,2-2,4 g/cm³ | ||
|MolVolume = | |MolVolume = 74,69 cm<sup>3</sup>/mol | ||
|Molweight = | |Molweight = 172,17g /mol | ||
|Transparency = | |Transparency = | ||
|Cleavage = | |Cleavage = | ||
|Crystal_Habit = | |Crystal_Habit = | ||
|Twinning = | |Twinning = | ||
|Refractive_Indices = | |Refractive_Indices = α = 1,519-1,521<br>β = 1,522-1,523<br>γ = 1,529-1,530 | ||
|Birefringence = | |Birefringence = Δ = 0,010 | ||
|optical_Orientation = | |optical_Orientation = biaxial positive | ||
|Pleochroism = | |Pleochroism = | ||
|Dispersion = | |Dispersion = 58° | ||
|Phase_Transition = | |Phase_Transition = | ||
|chemBehavior = | |chemBehavior = | ||
| Line 30: | Line 30: | ||
back to [[Sulphate]] | back to [[Sulphate]] | ||
==Weblinks== | == Salt and salt-induced damages == | ||
<references/> | |||
=== on objects === | |||
<gallery caption="" widths="200px" heights="150px" perrow="3"> | |||
Image:Perleberg 14.04.2003 (50)-Ausschnitt.jpg|brick damged by gipsum, St. Jakobi Perleberg | |||
Image:| | |||
Image:| | |||
</gallery> | |||
<br clear=all> | |||
=== under the polarising microscope === | |||
<gallery caption="thin-section samples" widths="200px" heights="150px" perrow="3"> | |||
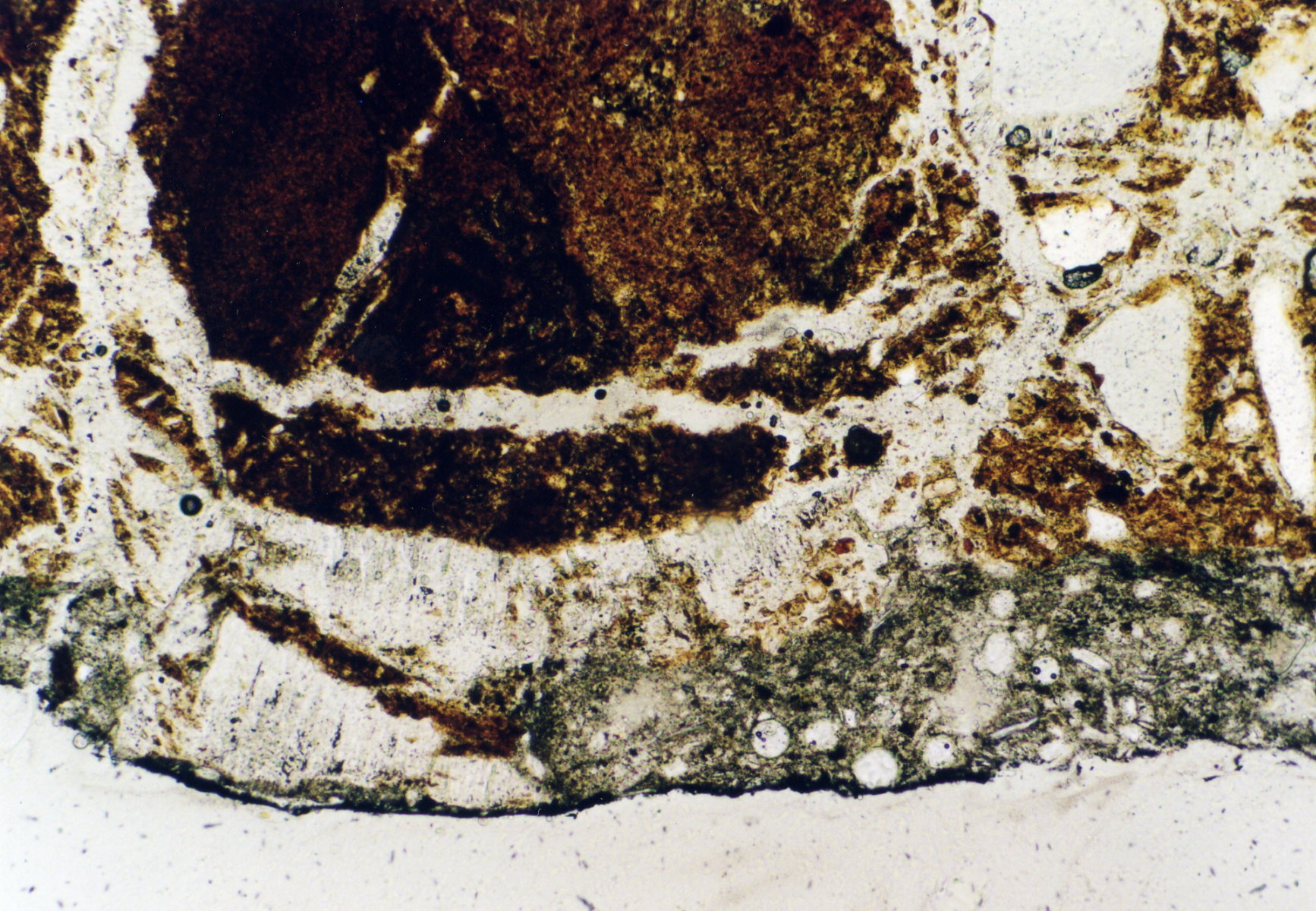
Image:Per_270603_5-13_14.jpg|brick damged by gipsum, St. Jakobi Perleberg | |||
Image:Per_270603_5-13_15.jpg|brick damged by gipsum, St. Jakobi Perleberg | |||
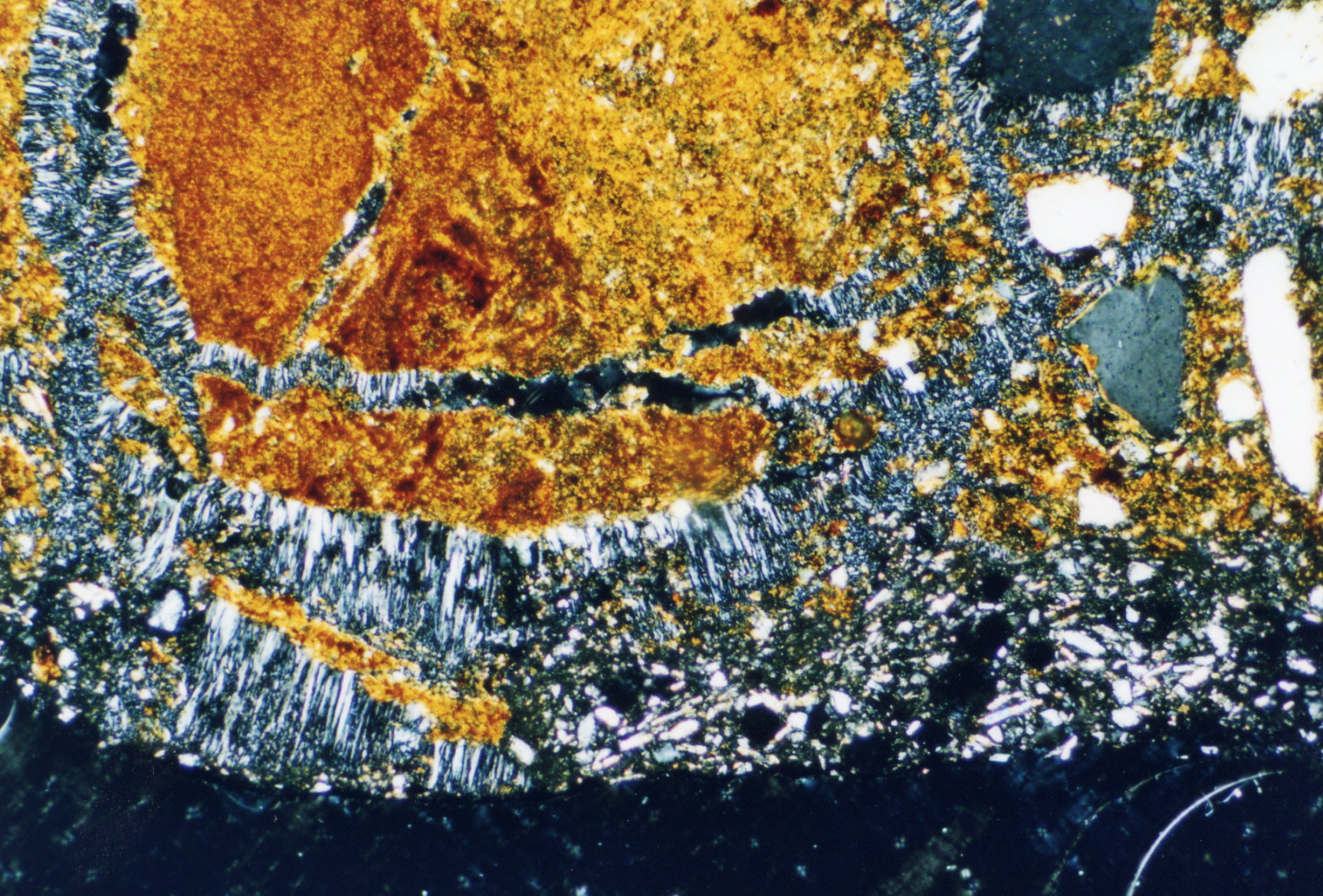
Image:Eilsum_Gips_duennschliff.jpg | |||
</gallery> | |||
<gallery caption="aqueous solution crystallised on a glass slide" widths="200px" heights="150px" perrow="3"> | |||
Image:CaSO4 pol 400x 01.JPG|Gipsum, crystallised from an aqueous solution on a glass slide | |||
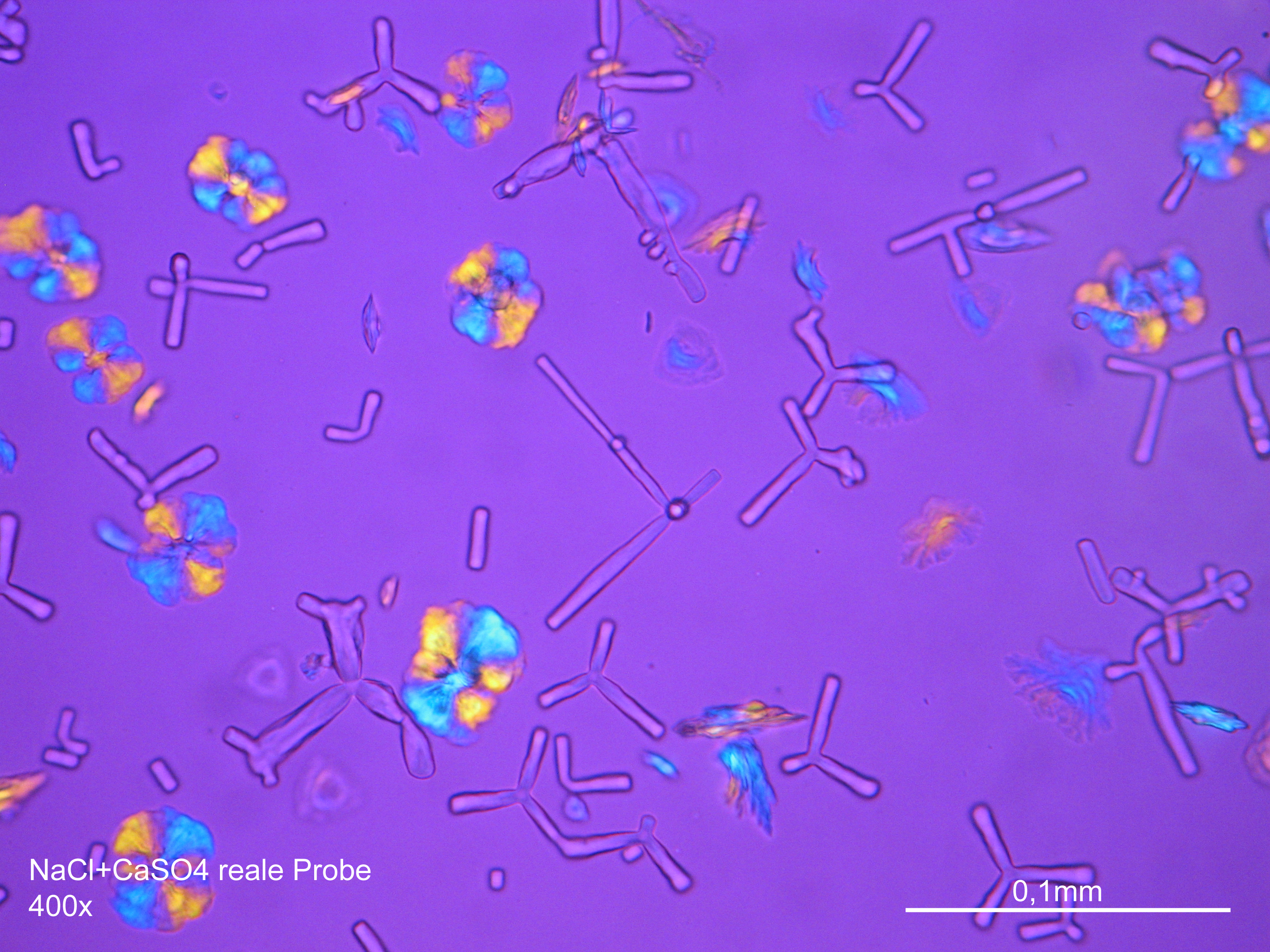
Image:CaSO4+NaCl reale Probe 01.JPG|Gipsum with Natriumchloride in a real sample, crystallised from an aqueous solution on a glass slide | |||
Image:CaSO4+NaCl reale Probe 02.JPG|Gipsum with Natriumchloride in a real sample, crystallised from an aqueous solution on a glass slide | |||
Image:HJS CaSO4 092503-1.jpg|Gipsum, crystallised from an aqueous solution on a glass slide | |||
</gallery> | |||
<br clear=all> | |||
== Under the polarising microscope == | |||
<gallery caption="SEM" widths="200px" heights="150px" perrow="3"> | |||
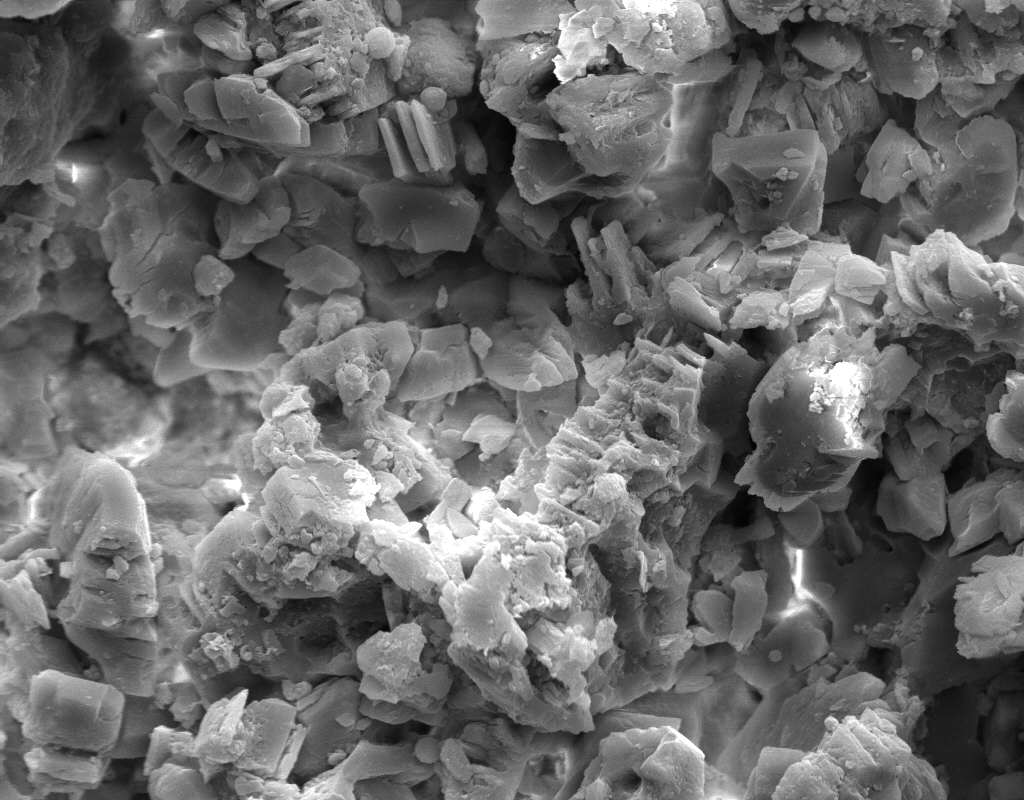
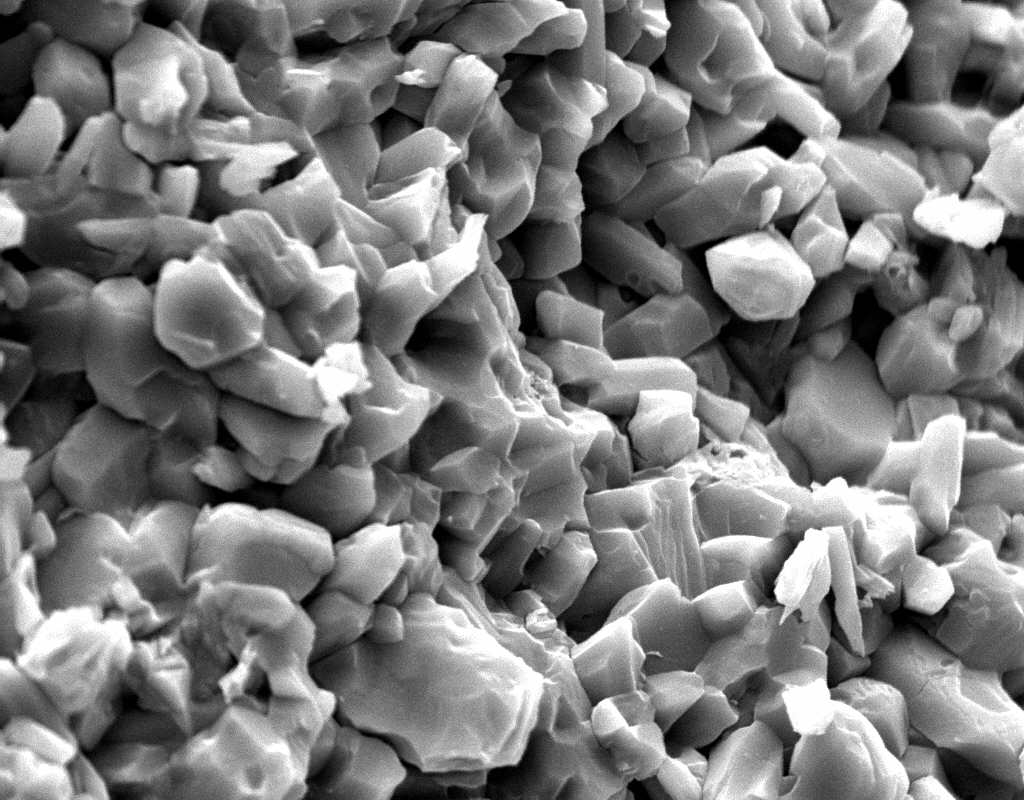
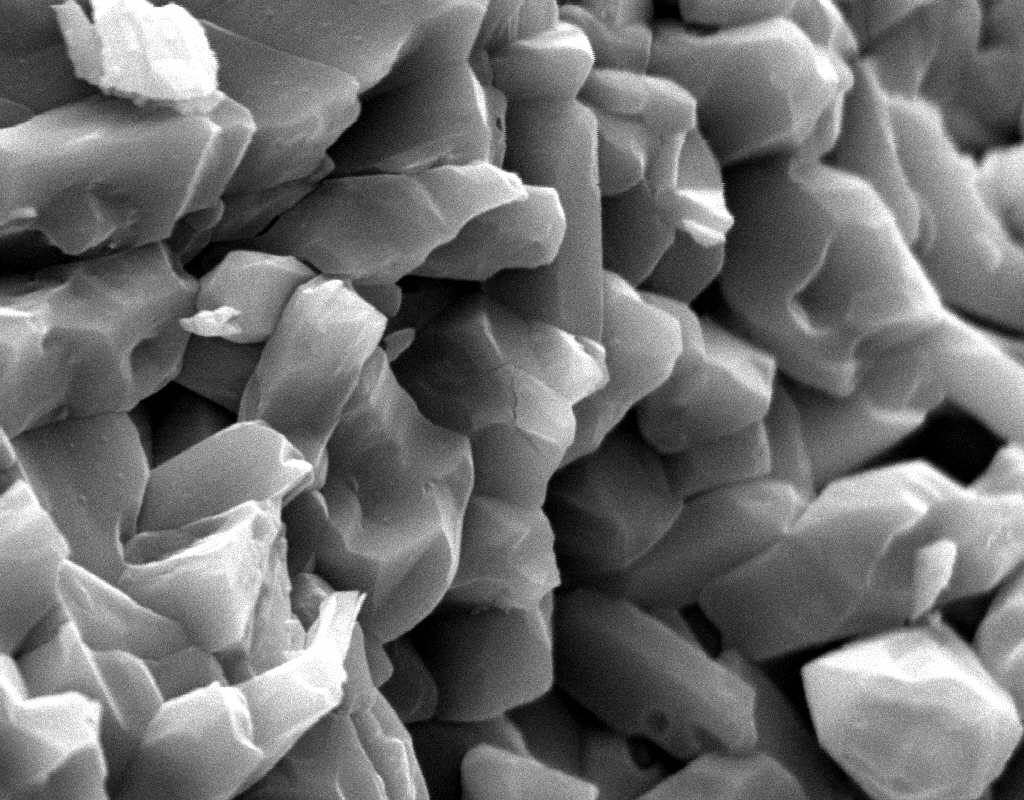
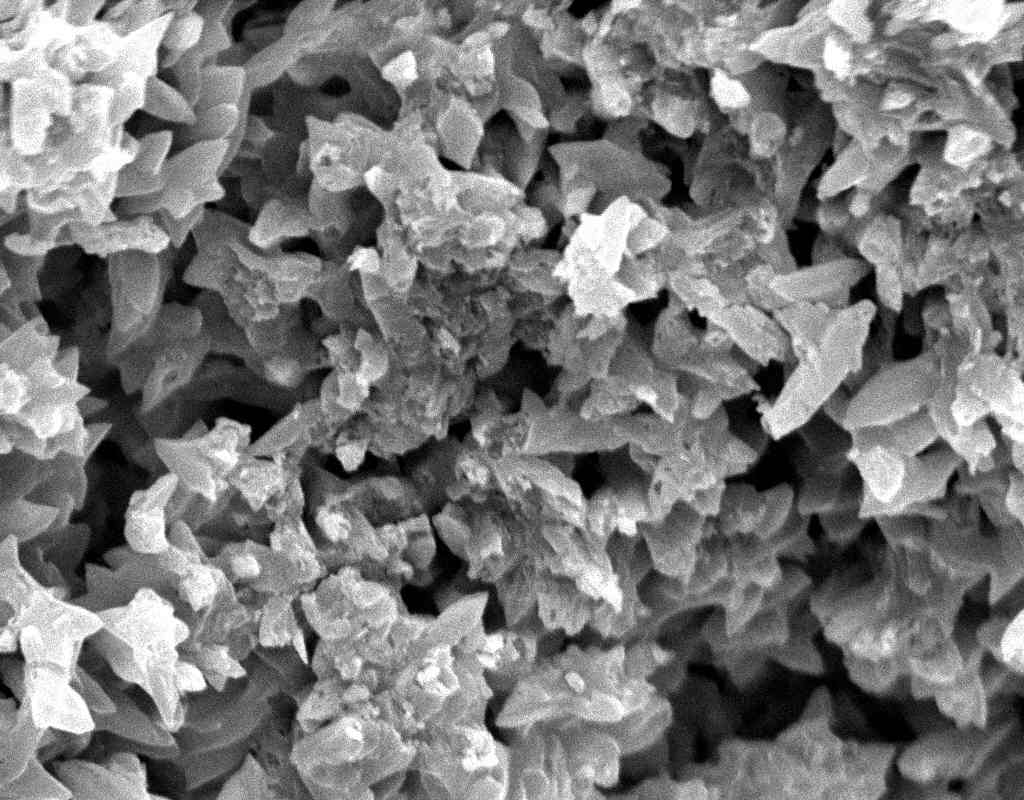
Image:SA100_1.jpg | Gipsum crystals in the SEM | |||
Image:SG2-2.jpeg | Gipsum crystals in the SEM | |||
Image:SG2-3.jpg | Gipsum crystals in the SEM | |||
Image:SG3-SPC2.jpeg | Gipsum crystals in the SEM | |||
Image:SG3-3.jpeg | Gipsum crystals in the SEM | |||
Image:SG3-4.jpeg | Gipsum crystals in the SEM | |||
Image:SG1-5.jpeg | Gipsum crystals in the SEM | |||
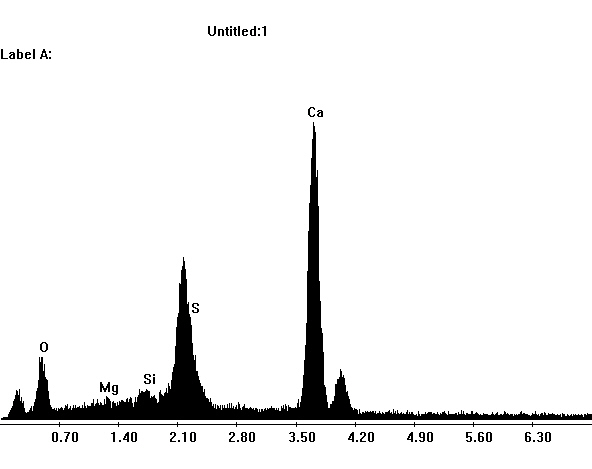
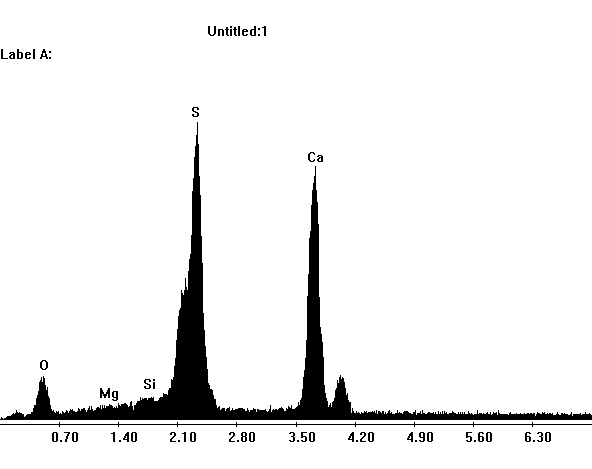
Image:SG1-SPC.jpeg | EDX spectrum of gypsum in the SEM | |||
</gallery> | |||
<br clear=all> | |||
== Weblinks == | |||
<references /> | |||
[[Category:Gipsum]][[Category:Sulphate]][[Category:Salt]][[Category:InProgress]] | [[Category:Gipsum]][[Category:Sulphate]][[Category:Salt]][[Category:InProgress]] | ||
Revision as of 14:45, 3 June 2011
| Gypsum[1][2] | |
| SA101 1.jpeg | |
| Mineralogical name | Gipsum, Selenite |
| Chemical name | Calcium sulphate dihydrate |
| Trivial name | Gypsite, Sulphate of Lime |
| Chemical formula | Ca[SO4]•2H2O |
| Other forms | Anhydrite (CaSO4) Hemihydrate (CaSO4•0,5H2O) |
| Crystal system | {{{Crystal_System}}} |
| Crystal structure | |
| Deliquescence humidity 20°C | |
| Solubility (g/l) at 20°C | |
| Density (g/cm³) | 2,2-2,4 g/cm³ |
| Molar volume | 74,69 cm3/mol |
| Molar weight | 172,17g /mol |
| Transparency | |
| Cleavage | |
| Crystal habit | |
| Twinning | |
| Phase transition | |
| Chemical behavior | |
| Comments | |
| Crystal Optics | |
| Refractive Indices | α = 1,519-1,521 β = 1,522-1,523 γ = 1,529-1,530 |
| Birefringence | Δ = 0,010 |
| Optical Orientation | biaxial positive |
| Pleochroism | |
| Dispersion | 58° |
| Used Literature | |
| {{{Literature}}} | |
back to Sulphate
Salt and salt-induced damages[edit]
on objects[edit]
under the polarising microscope[edit]
- thin-section samples
- aqueous solution crystallised on a glass slide
Under the polarising microscope[edit]
- SEM
Weblinks[edit]
- ↑ http://webmineral.com/data/Gypsum.shtml seen on 30.07.2010
- ↑ http://www.mindat.org/min-1784.html seen on 30.07.2010