Niter
<bibimport/>
Authors: Hans-Jürgen Schwarz, Nils Mainusch, NN....
Translation of the German Version by Hans-Jürgen Schwarz
back to Nitrate
| Niter[1][2][3] | |
| |
| Mineralogical name | Nitrokalite, Niter, Kalisalpeter |
| Chemical name | Potassium nitrate |
| Trivial name | Saltpetre , Nitrate of potash, Vesta powder, Kali-Salpeter, Kehrsalpeter, Konversionssalpeter |
| Chemical formula | KNO3 |
| Other forms | none |
| Crystal system | orthorhombic |
| Crystal structure | orthorhombic - dipyramidal; 2/m 2/m 2/m , see [4] |
| Deliquescence humidity 20°C | 94.6 % (20°C), 93.6% (25°C) |
| Solubility (g/l) at 20°C | 315 g/l |
| Density (g/cm³) | 2.109 g/cm3 |
| Molar volume | 48.04 cm3/mol |
| Molar weight | 101.11 g/mol |
| Transparency | translucent to transparent |
| Cleavage | very good on {001}; good on {010}h[5] |
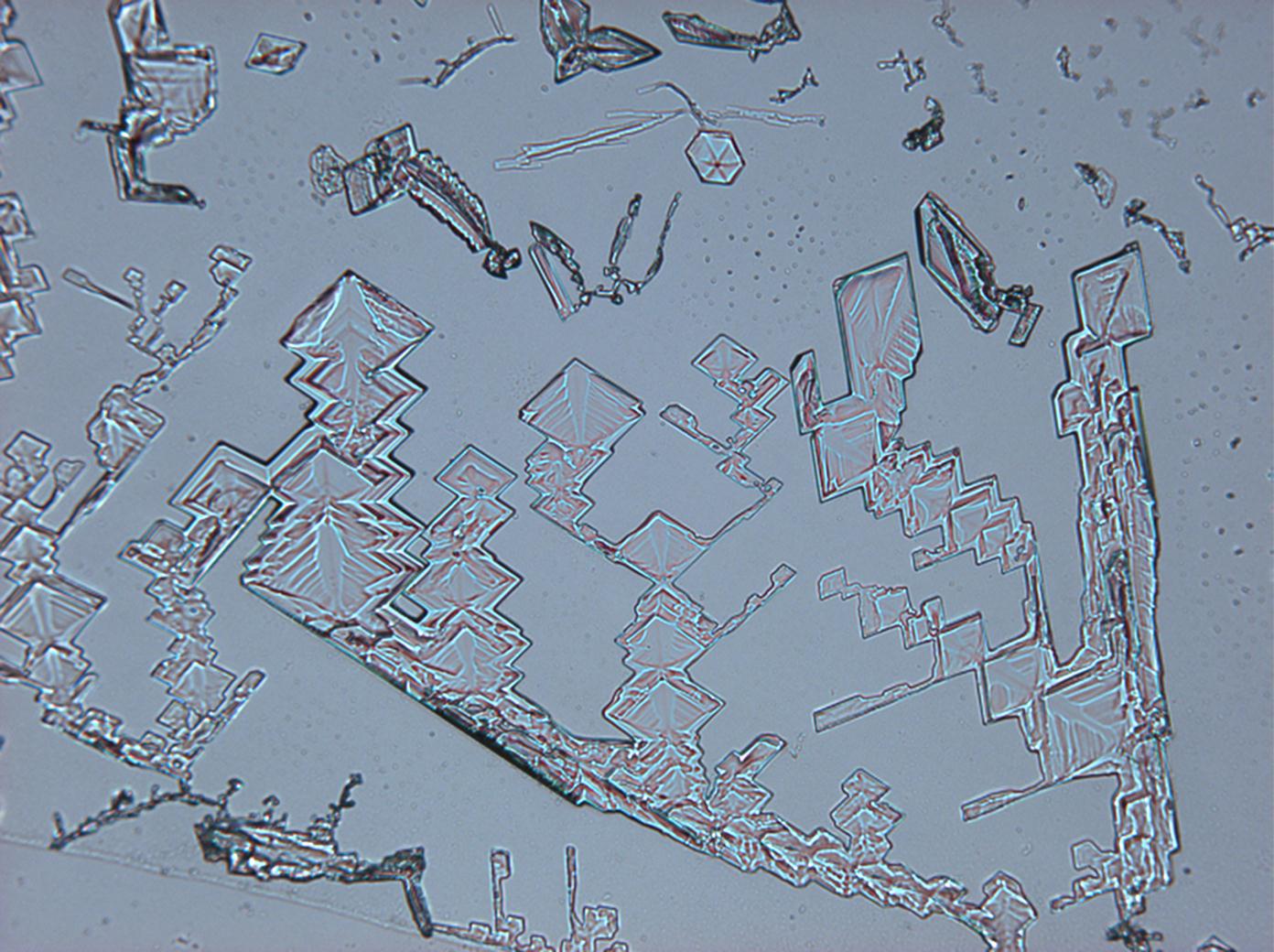
| Crystal habit | include crusts and acicular crystals formed as efflorescence on cave and mine walls [6] |
| Twinning | |
| Phase transition | |
| Chemical behavior | easily soluble in water |
| Comments | |
| Crystal Optics | |
| Refractive Indices | α = 1.335 β = 1.505 γ = 1.506 |
| Birefringence | Δ = 0.171 |
| Optical Orientation | biaxial negative |
| Pleochroism | |
| Dispersion | weak, r < v |
| Used Literature | |
| {{{Literature}}} | |
Abstract[edit]
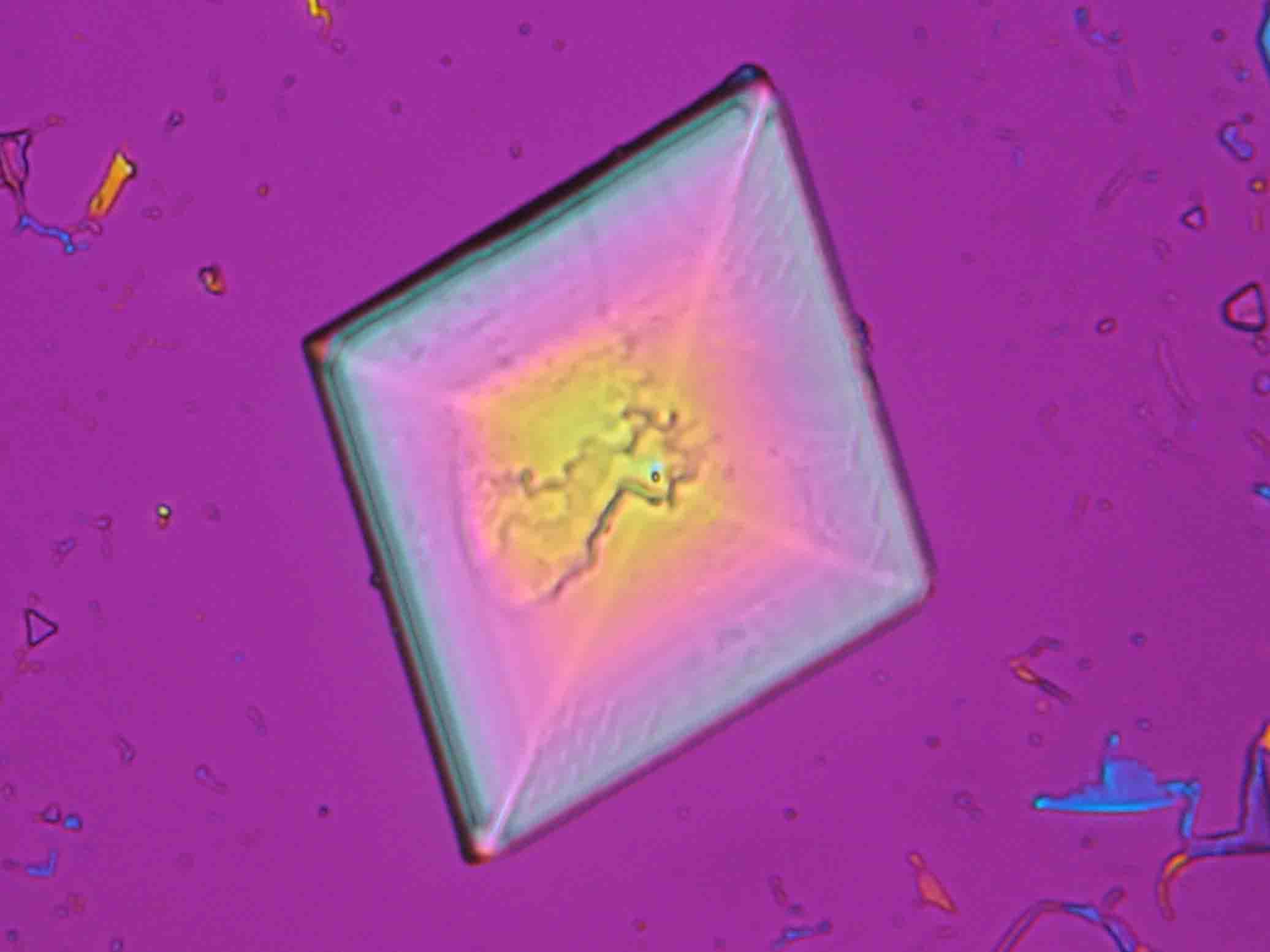
Niter is one of the most important salts that is responsible for damages of buildings and murals. He is mostly observed indoors, often found in cotton-like efflorescences. Pictures, microphotographs and examples of habits illustrate and complement the description.
General[edit]
Potassium salts were an important ingredient for the production of gun and explosives in the 19th century, and were both originating from natural sources (potash deposits contain a maximum of 10% KNO3 among various other potassium salts) and obtained through the transformation of sodium nitrate into potassium chloride. Currently various methods of large-scale production of this salt are based on the reaction between crude potassium salts and nitric acid.
Potassium nitrate is also used as a fertilizer in agriculture.
Occurrence of Niter[edit]
Potassium nitrate in natural accumulations can occur anywhere where nitrogen compounds are synthesized in presence of a sufficient quantity of potassium ions (for example during the decomposition of organic matter). Large quantities of nitrifying bacteria and nitrogen compounds are known to be present in manure and urine of living organisms.
Information on the origin and formation of Niter on monuments[edit]
Contaminated ground water is the major input source of potassium ions in monuments, and nitrates most commonly come from atmospheric pollution. In addition, building and restoration materials can also contain soluble potassium compounds. We can also mention potassium silicate, potassium hydroxide (used as a cleaning agent) and cements.
Nitrates can also originate from biogenic sources and be transported into the material structure via capillary transport of moisture. Microbial activity is an important potential source of nitrates through nitrifying bacteria.
Solution behavior[edit]
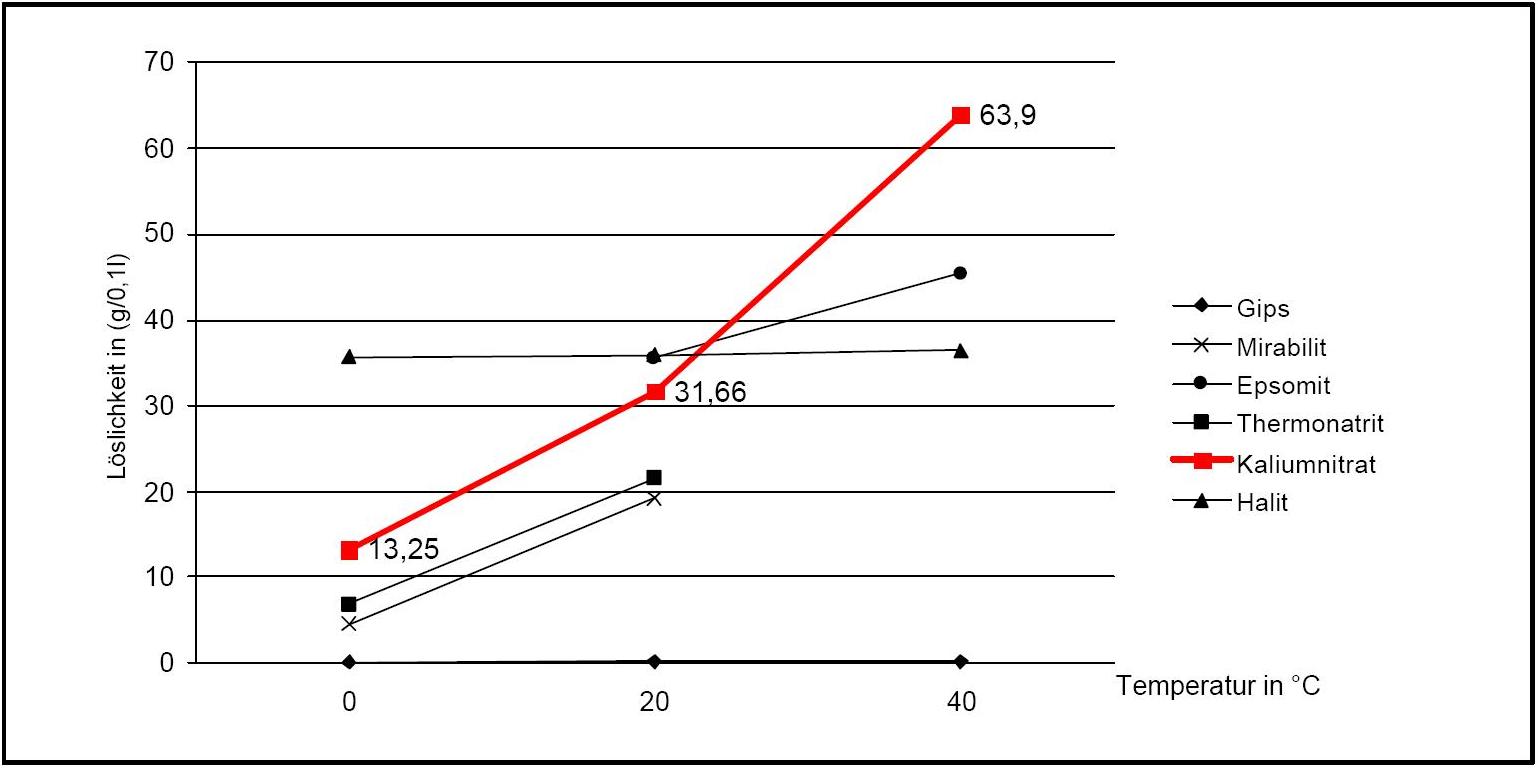
Author: Stark, Jochen; Stürmer, Sylvia
 )
)Potassium nitrate has a high water solubility, belonging to the group of mobile salts. It can be associated with frequent changes in the accumulation zones in the porous material. The temperature effect on the water solubility is strong, which is reflected in the steep curve in Diagram 1.
A consequence of this is the danger of solution supersaturation during rapid temperature drop, and a subsequent crystallization of the salt.
Mix up
Potassium nitrate is clearly identifiable. The data in the table below show salts which have refractive indices that are similar to those of potassium nitrate, however, there are clear distinguishing features.
| Salt Phase | Distinguishing Features to Niter |
| Nitronatrite NaNO3 | |
| Nitromagnesite Mg(NO3)2 • 6H2O | inclined extinction/ hygroscopic |
| Nitrocalcite Ca(NO3)2 • 4H20 | significantly lower birefringence/inclined extinction/hygroscopic |
Niter and damages caused by niter in the image[edit]
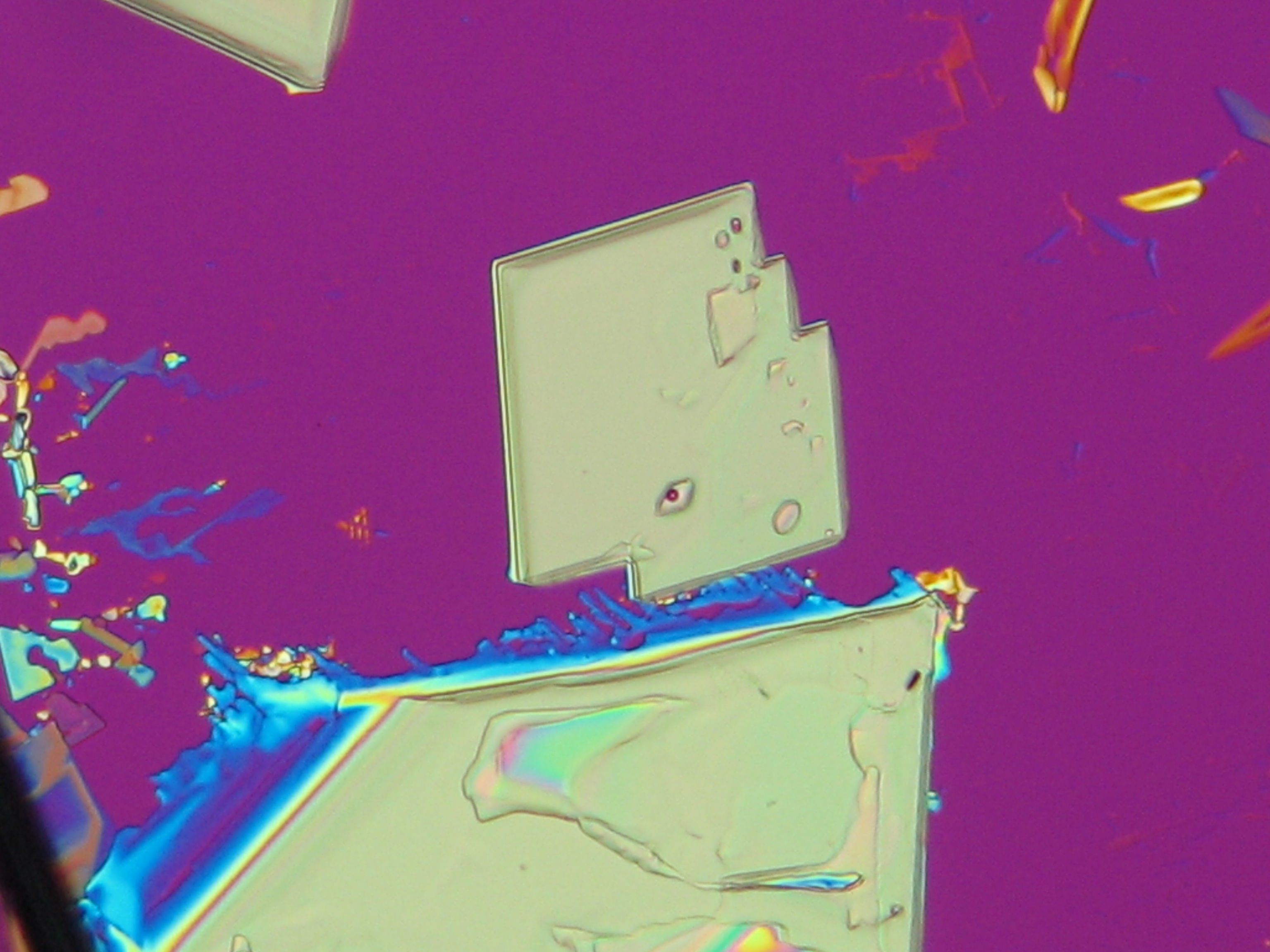
On objects[edit]
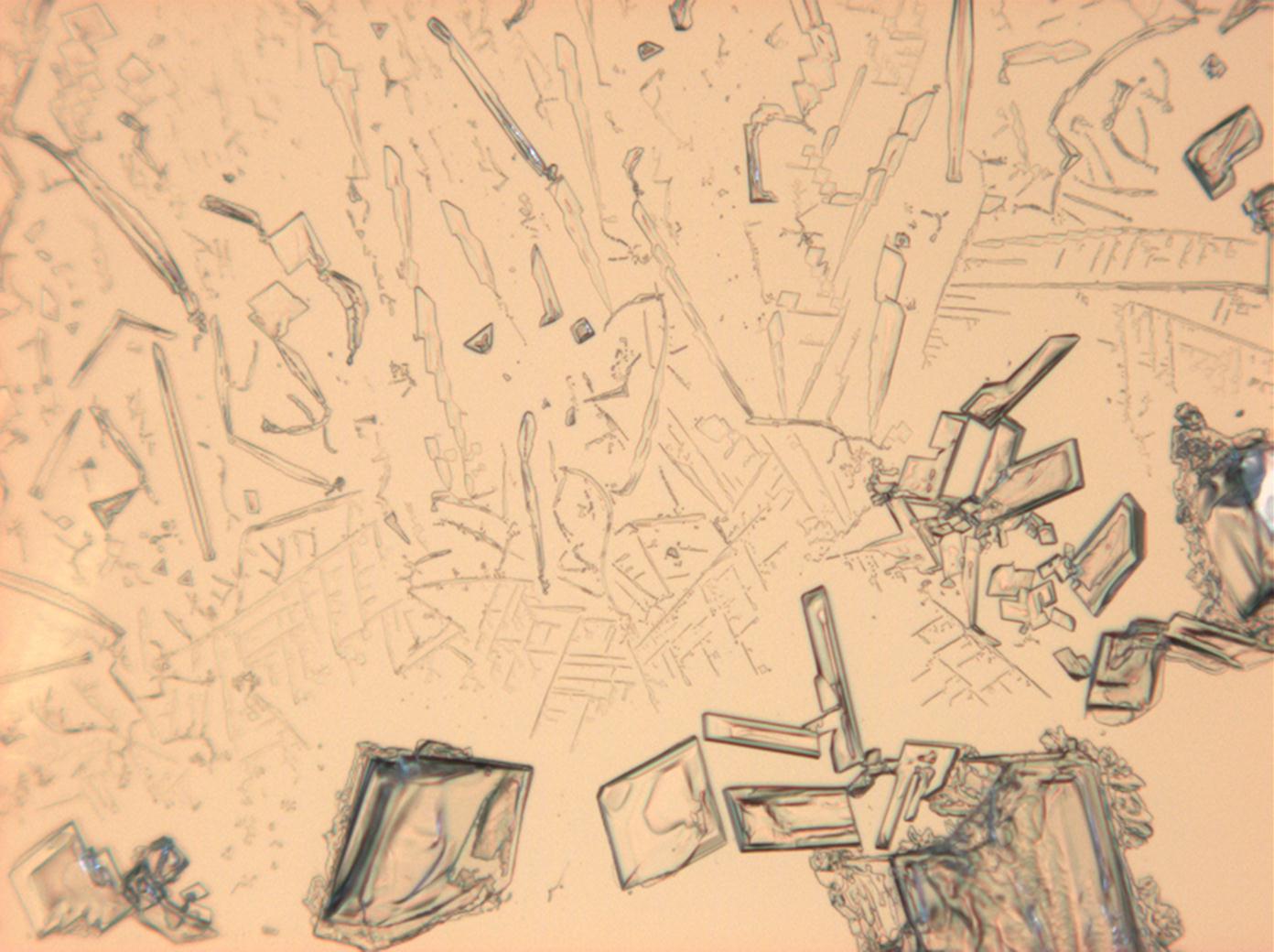
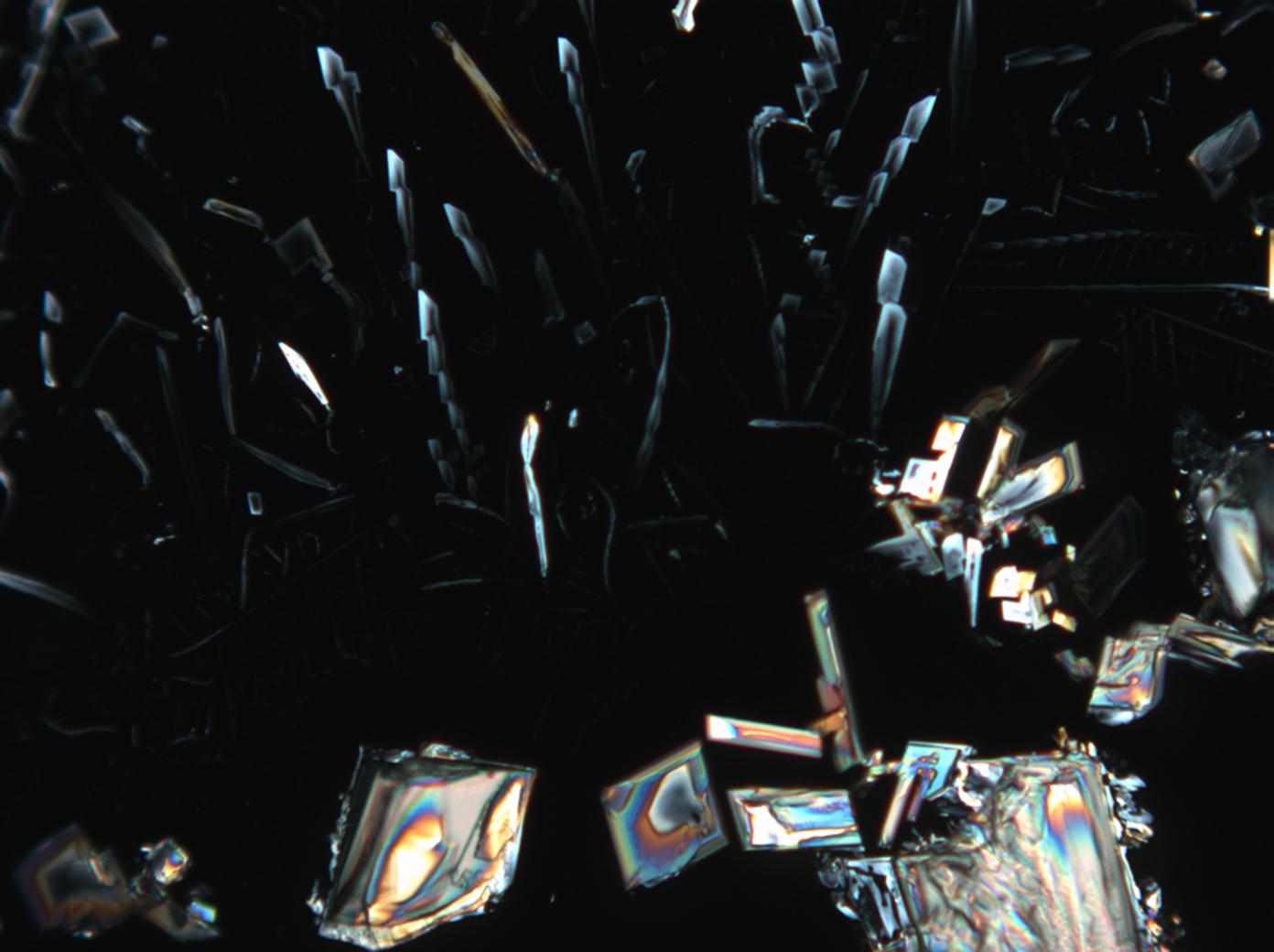
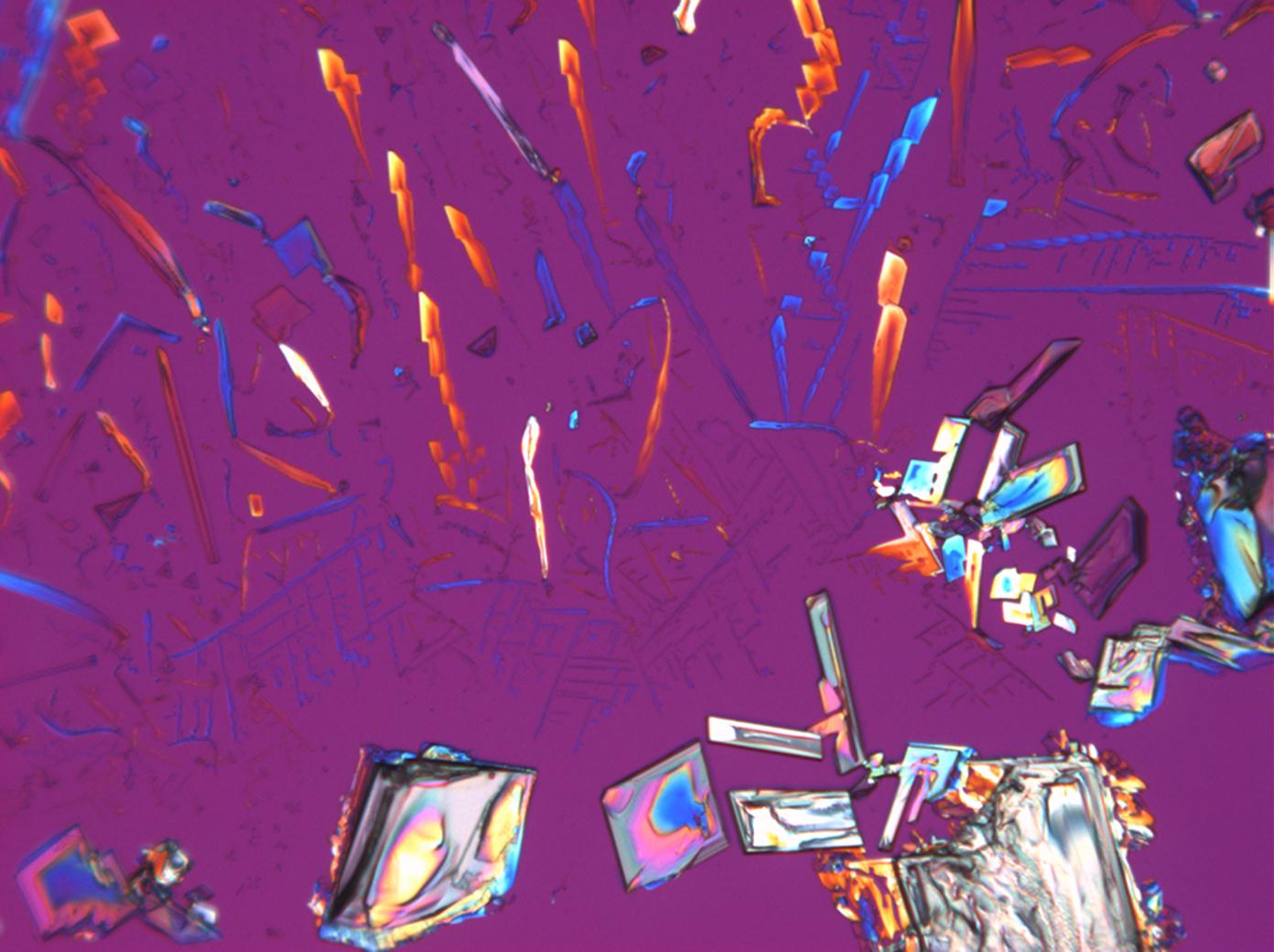
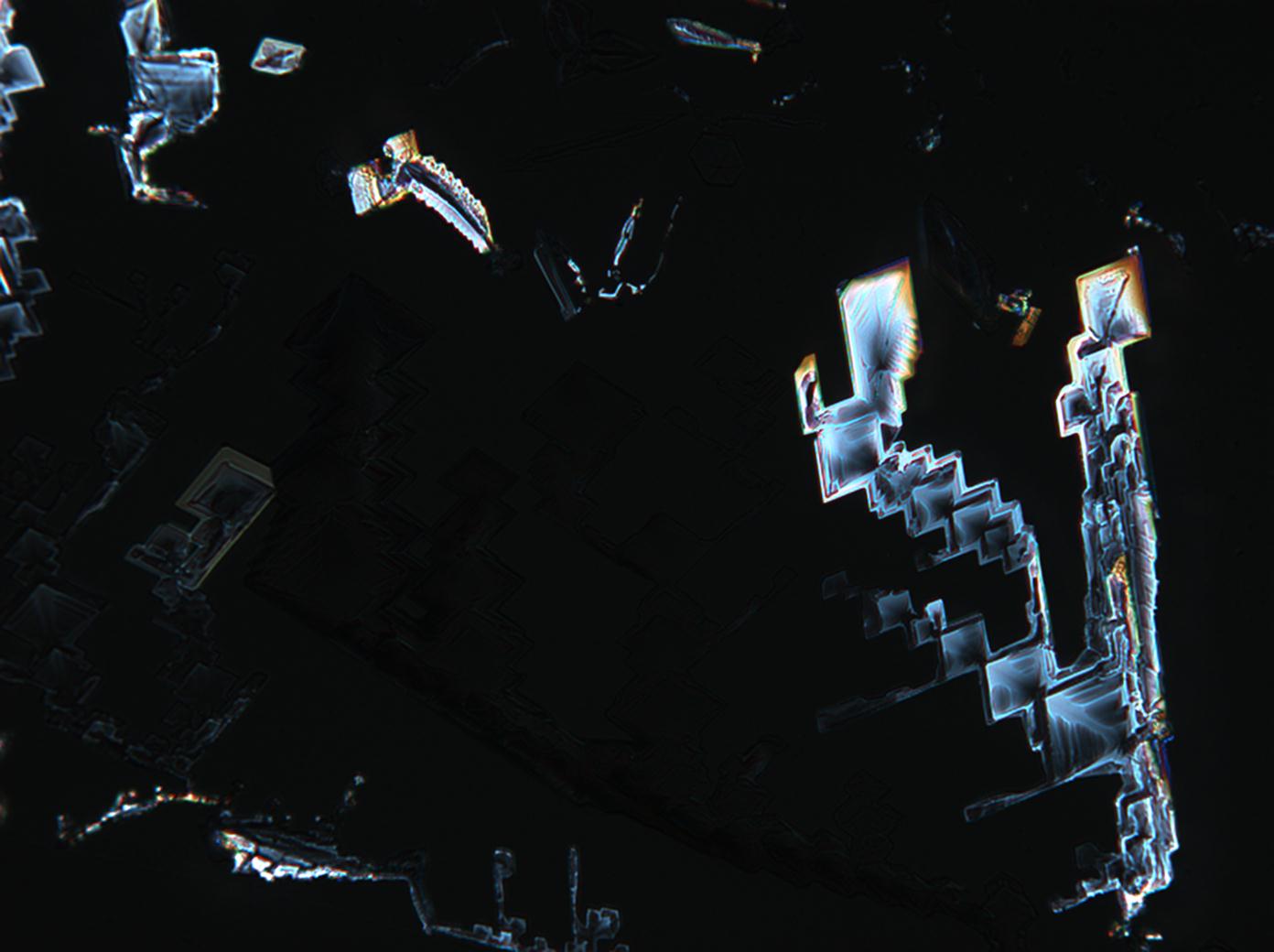
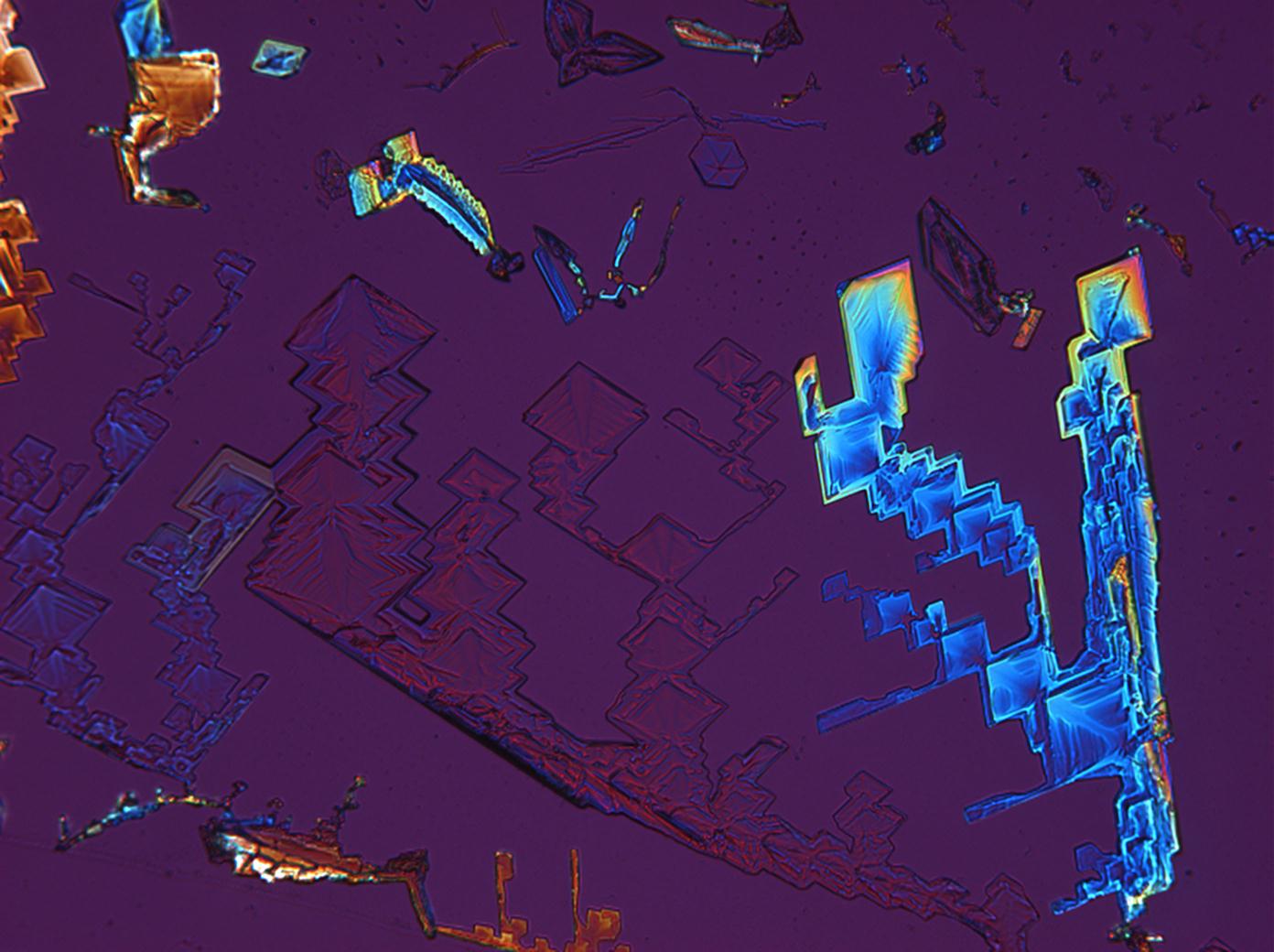
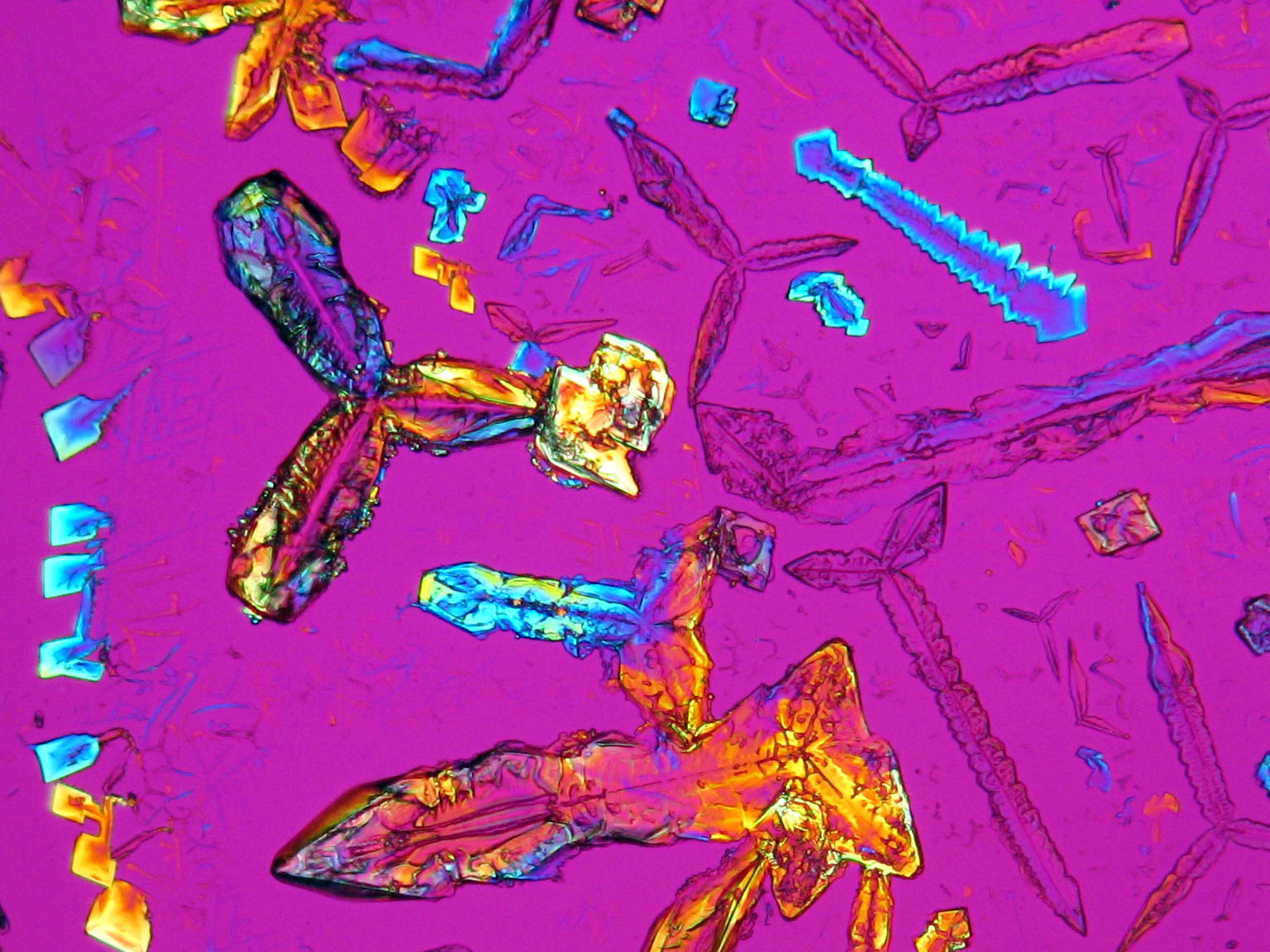
Under the polarizing microscope[edit]
- KNO<sub
Weblinks[edit]
- ↑ http://webmineral.com/data/Niter.shtml gelesen 28.07.2010
- ↑ http://www.mindat.org/min-2917.html
- ↑ http://www.mineralienatlas.de/lexikon/index.php/MineralData?mineral=Niter
- ↑ http://webmineral.com/jpowd/JPX/jpowd.php?target_file=Niter.jpx; Viewed on 15/04/2011
- ↑ ttp://en.wikipedia.org/wiki/Niter; Viewed on 15/04/2011
- ↑ http://www.galleries.com/minerals/carbonat/niter/niter.htm; Viewed on 15/04/2011
Literature[edit]
[Filter missing]