Magnesium sulfate
Author: Amelie Stahlbuhk
back to Sulfate
| This article will be released soon. |
Abstract[edit]
The different hydrated forms of magnesium sulfate and the behaviour concerning solubility and hygroscopicity will be presented.
Hydrated forms[edit]
Kieserite MgSO4•H2O
Sanderite MgSO4•2H2O
Starkeyite MgSO4•4H2O
Pentahydrite MgSO4•5H2O
Hexahydrite MgSO4•6H2O
Epsomite MgSO4•7H2O
Meridianiite MgSO4•11H2O
Solubility[edit]
As it is shown in table 1, the different hydrated forms of magnesium sulfate are easily soluble salts, which leads to a high mobility of the salts in porous materials.
| Hydrated form | Solubility [mol/kg] at 20°C |
| Kieserite | 5.60 |
| Starkeyite | 5.04 |
| Pentahydrite | 4.40 |
| Hexahydrite | 3.61 |
| Epsomite | 2.84 |
Due to the different hydrated forms of magnesium sulfate with stable and meta stable equilibria, the solubility diagram of the system MgSO4-H2O contains more information than diagrams of salts with less or also without any hydrated forms. With the temperature dependence of the solubility it is possible that temperature changes are accompanied by a hydration or a dehydration of a considered phase.
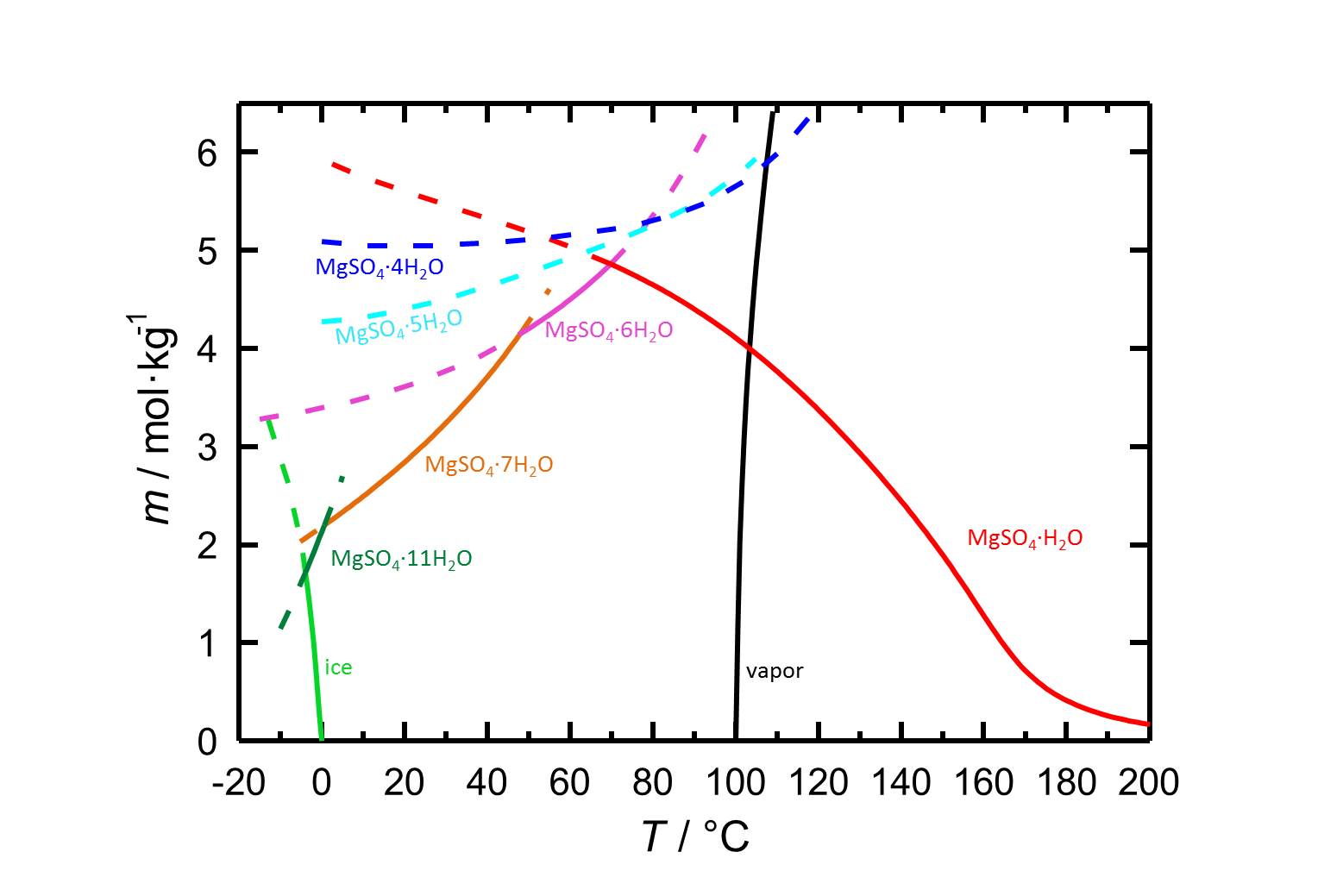
Author: Steiger, Michael; Linnow, Kirsten; Ehrhardt, Dorothee; Rohde, Mandy
 .
.
Hygroscopicity[edit]
In the system MgSO4-H2O changes in temperature or relative humidity may lead to hydration/dehydration or deliquescence/crystallization processes. At 20 °C epsomite is the present crystalline phase when the relative humidity is below its deliquescence humidity of 91.3 %. When the relative humidity reaches values below 47 % the dehydration to lower hydrated levels sets in, as it is represented by the curves of the equilibrium humidities in figure 2.
| Phase transition | Deliquescence or equilibrium humidity at 20°C |
| Epsomite-solution | 91.3 % |
| Epsomite-Hexahydrite | 46.6 % |
| Epsomite-Kieserite | 46.7 % |
| Hexahydrite-Starkeyite | 39.1 % |
In the temperature range of -10 to 100 °C the deliquescence humidities of the present hydrated forms (depending on the temperature) lie always above 80 % r.h., so the salts do not belong to the hygroscopic salts.
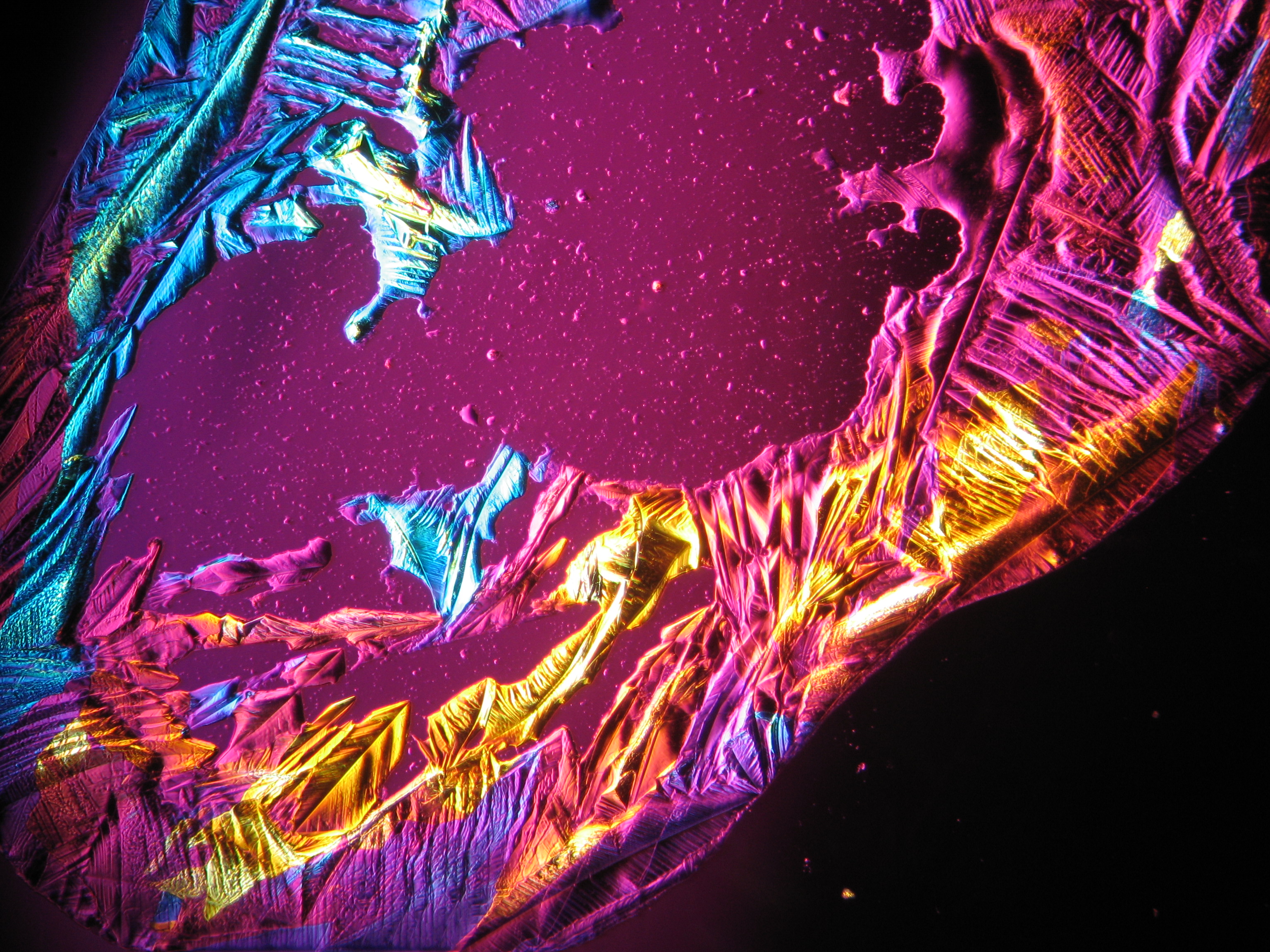
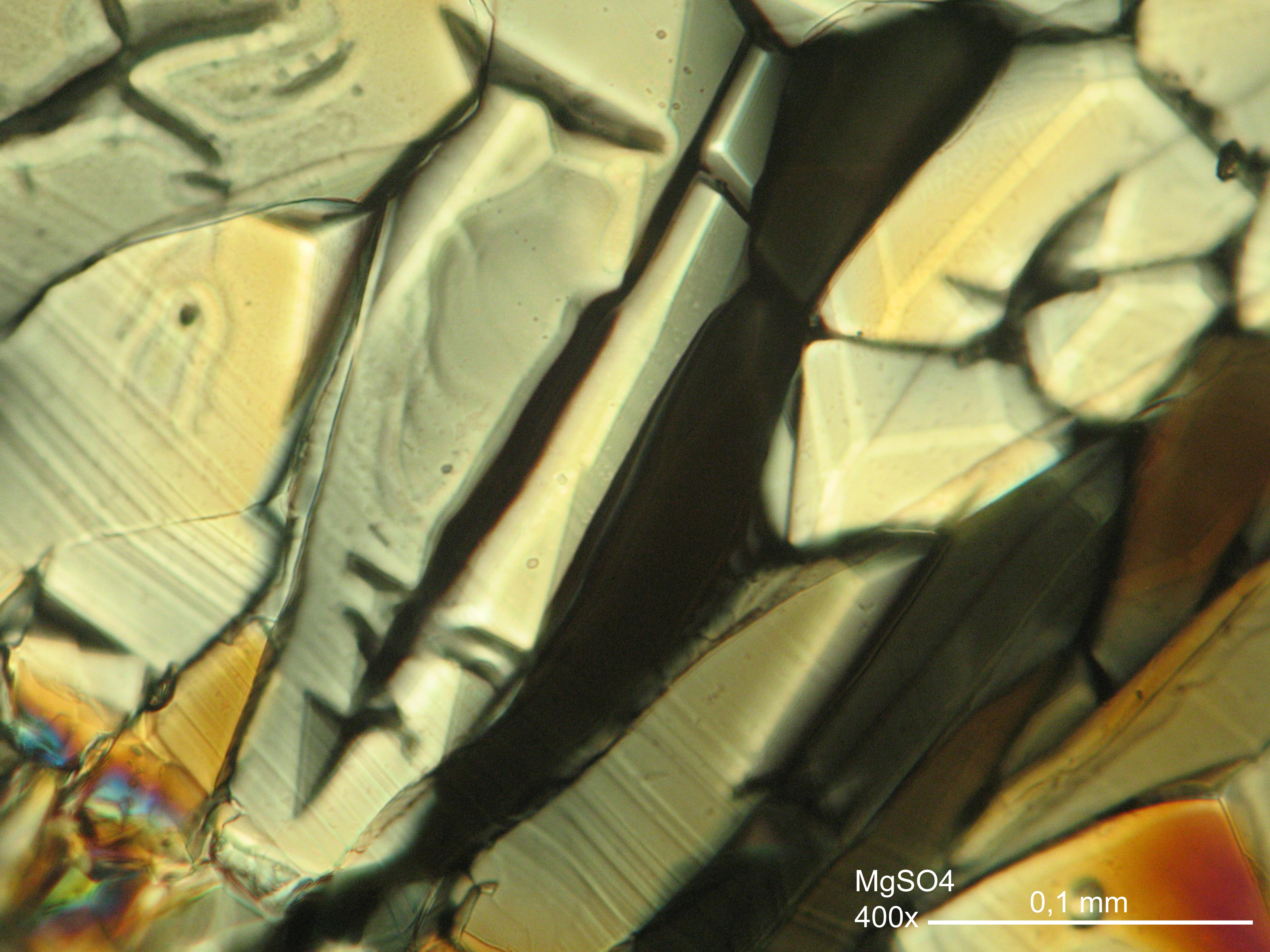
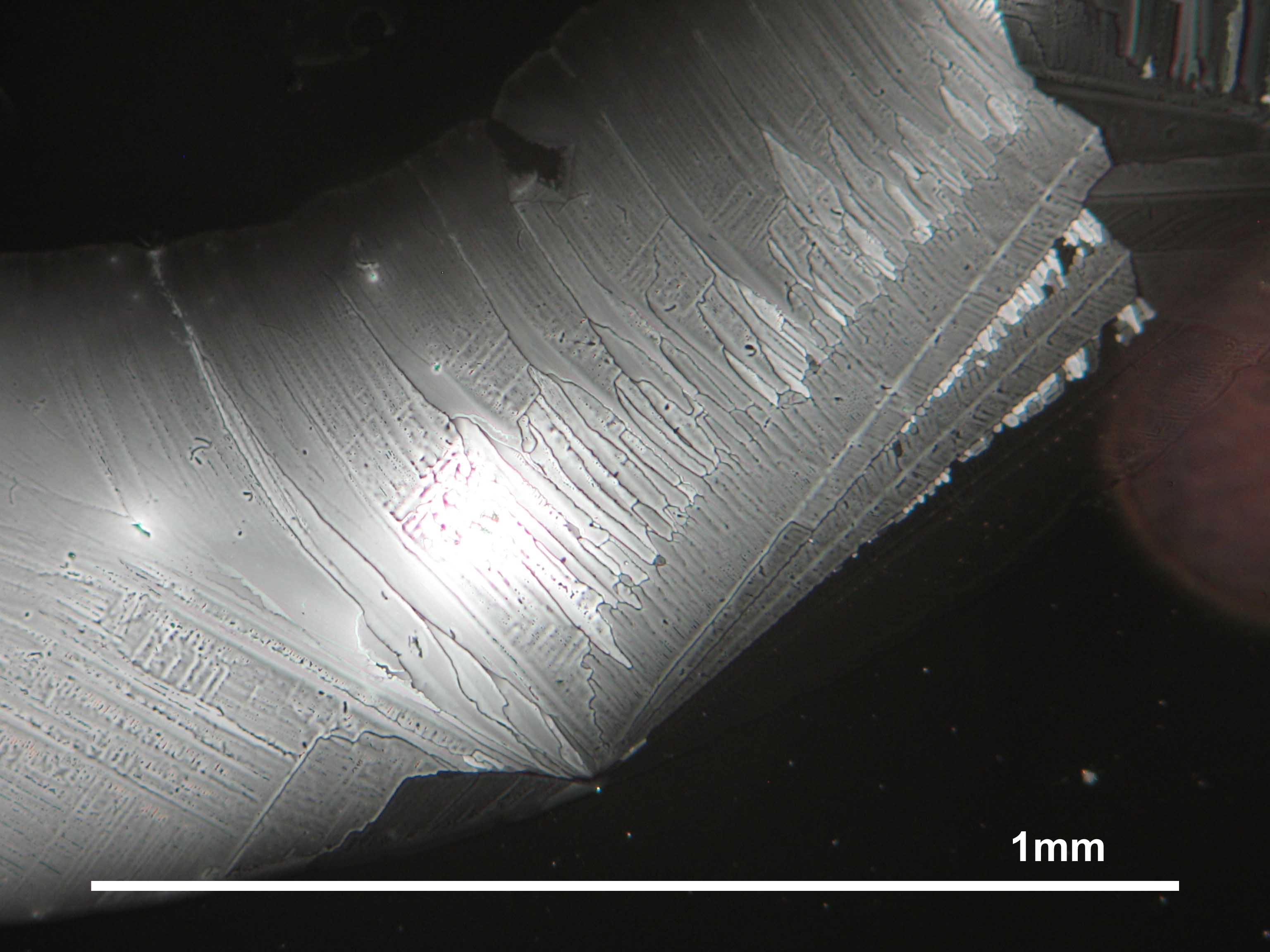
Microscopy[edit]
Laboratory examination:
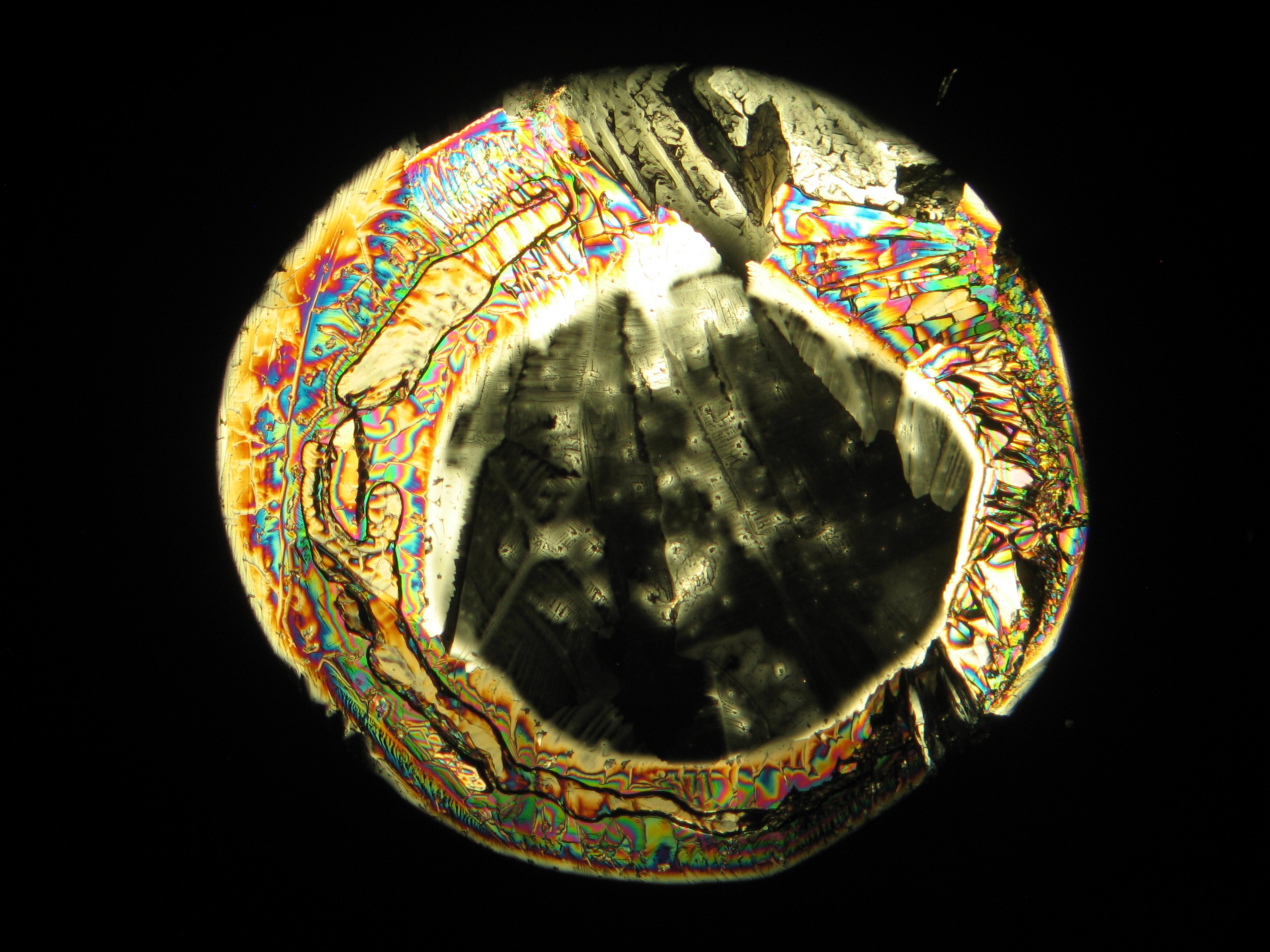
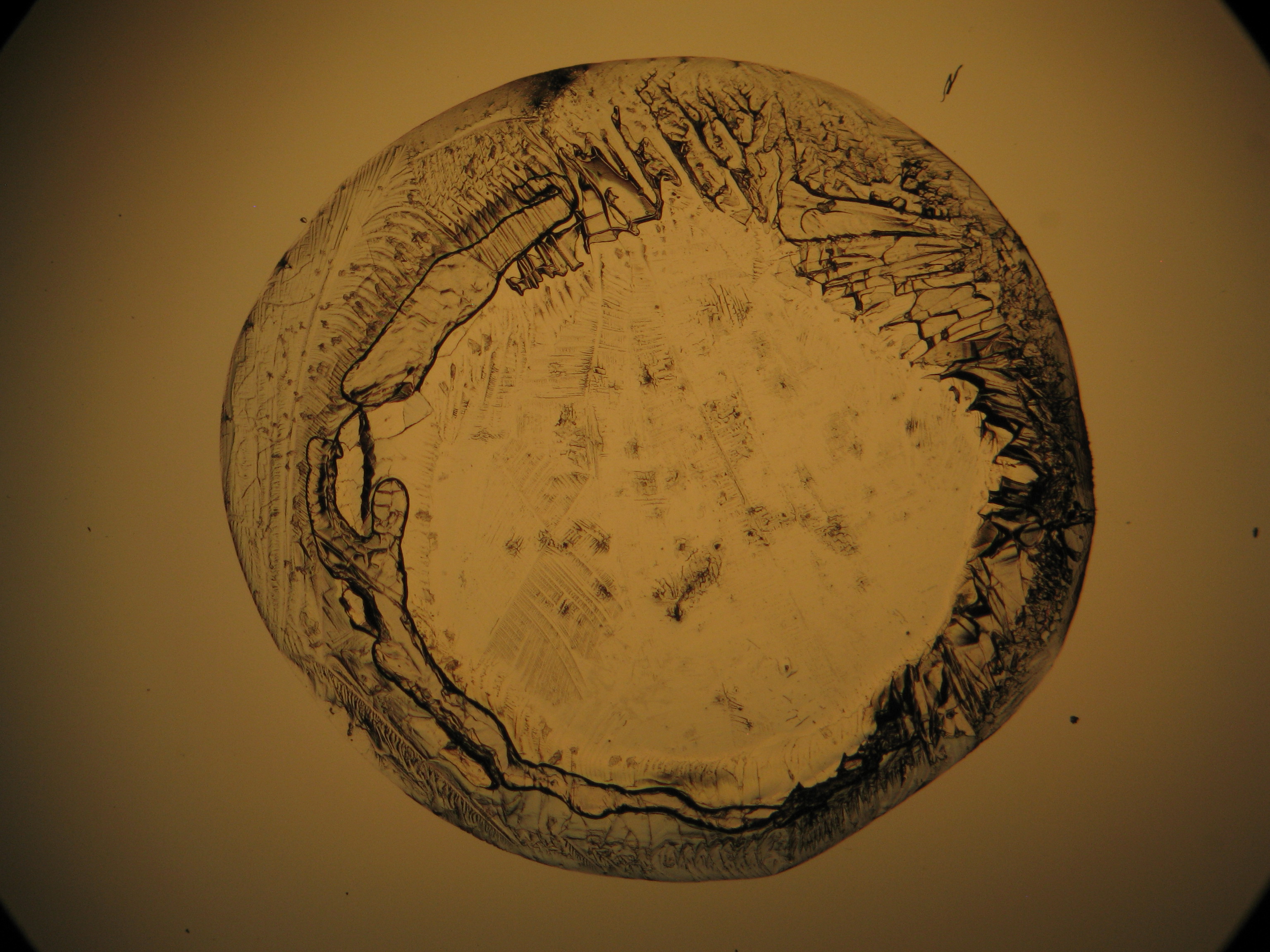
Breathing onto a (predominantly) magnesium sulfate efflorescence, causes no observable macroscopic changes. However, characteristics are: the good solubility in water, a pH of 7, the formation of a ring-shaped slightly raised seam (visible by the naked eye), or a transparent layer that remains in place after the evaporation of the aqueous solvent.
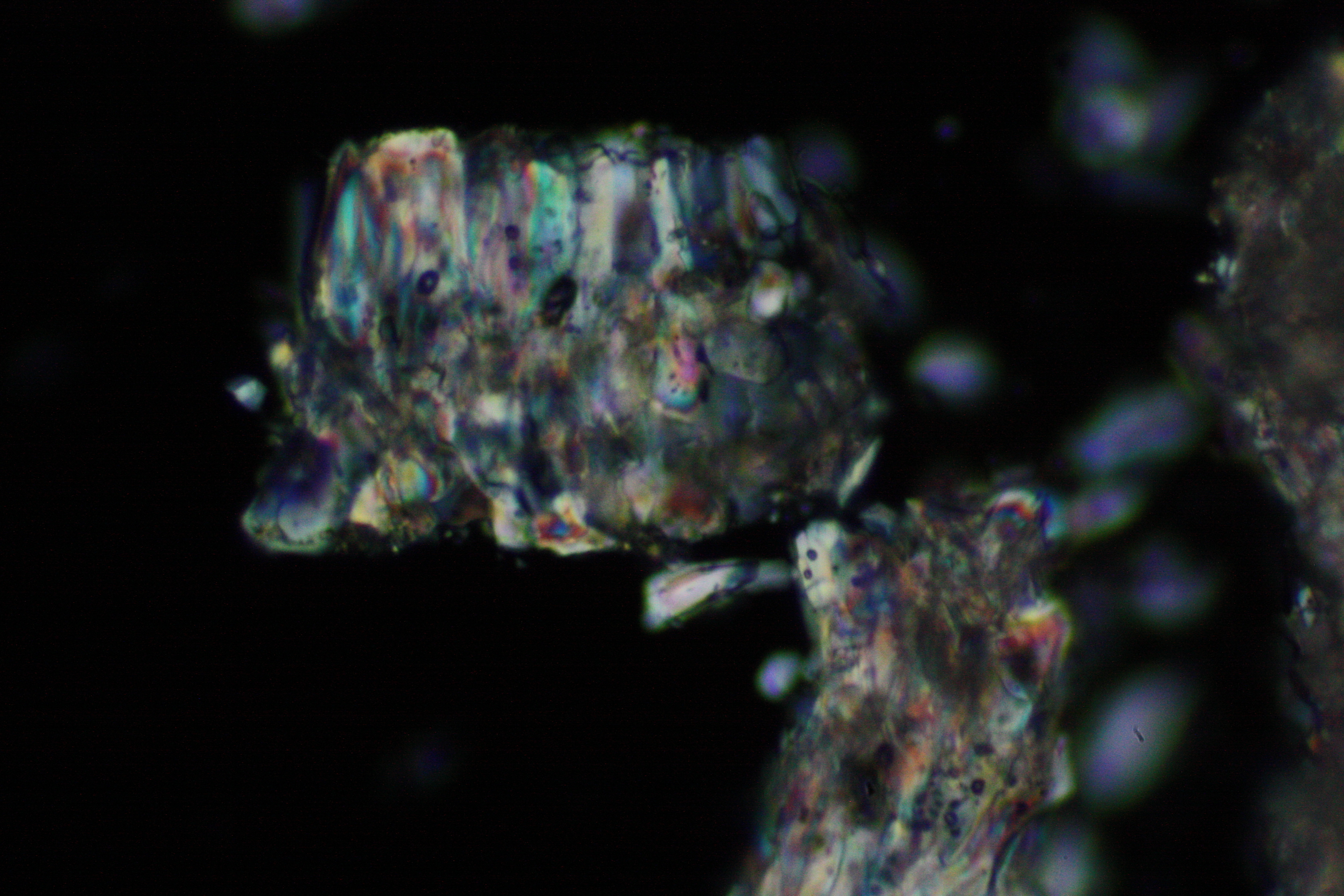
In laboratory tests on objects the results should be double checked with the microscope. Observations of the raw sample material and the recrystallized salt, show that magnesium sulfate crystals appear in unspecific forms in sample material.
Experiments to produce well-shaped, single magnesium sulfate crystals, by recrystallization in aqueous solution have not been very successful, because of a strong tendency for intergrowth. Usually the previously mentioned, distinct, ring-shaped seam of intergrown crystals forms, when the magnesium sulfate containing solute is evaporated. Epsomite shows a low solubility in anhydrous ethanol and glycerin under the microscope.
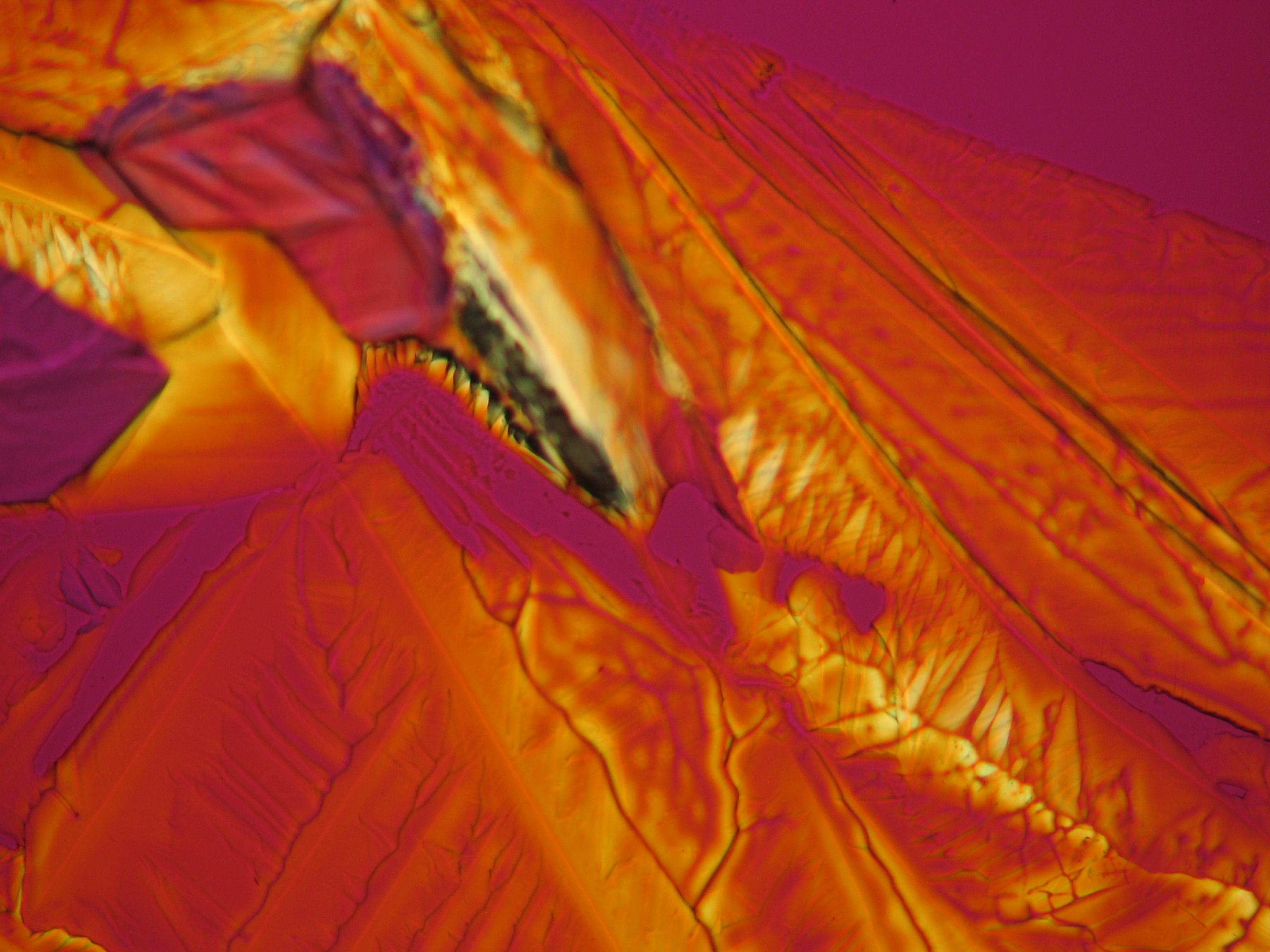
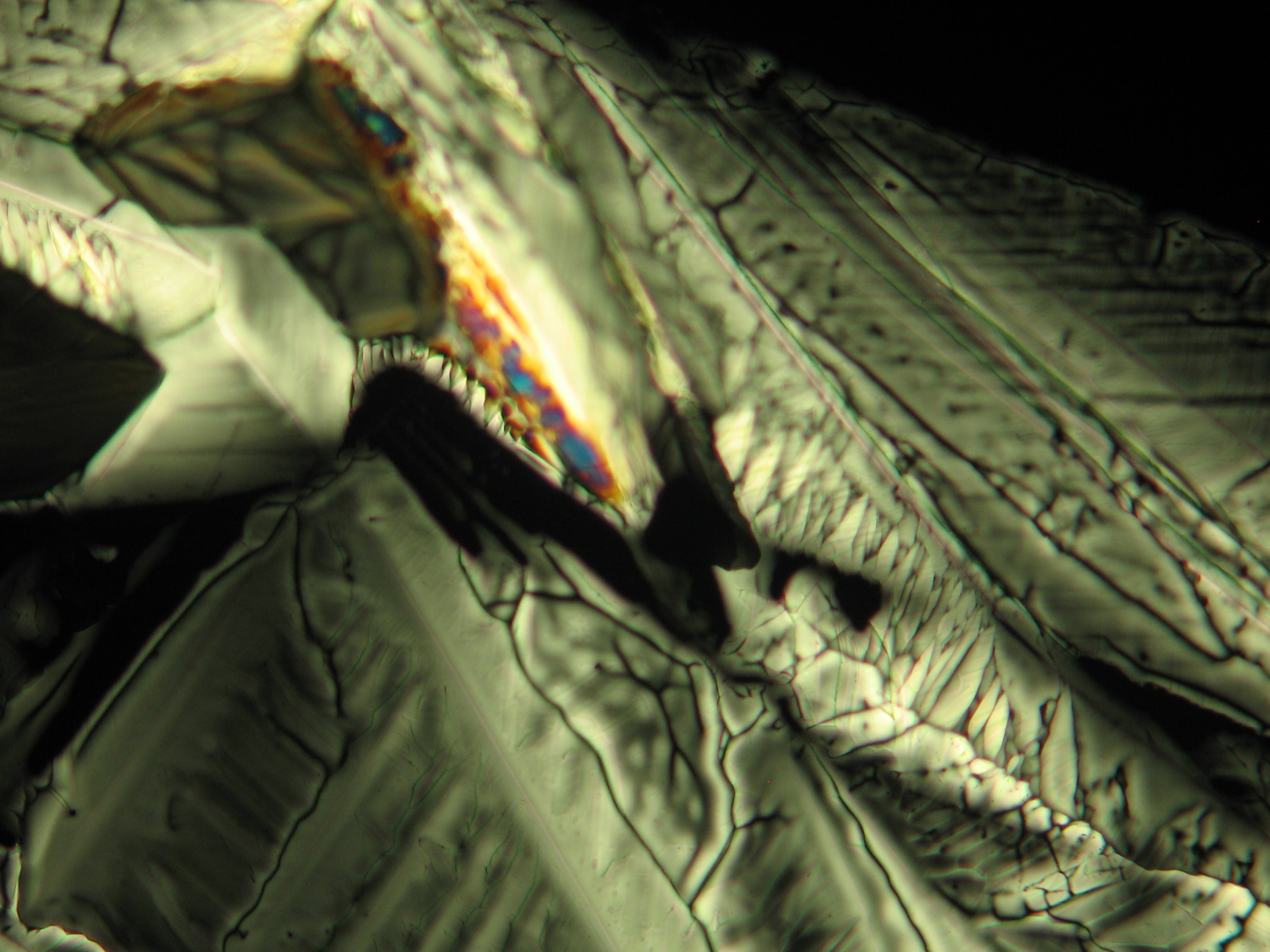
For further examinations using polarized light microscopy, a considerable difficulty is the creation of good preparations, due to the tendency of magnesium sulfate to form only few equidimensional or elongated single crystals. The low solubility in ethanol, nevertheless allows to isolate single particles by repeatedly adding recrystallized material. Both on the base material as well as the recrystallized product, examination using polarized light microscopy should be carried out.
Refractive indices: nX = 1,433, ny = 1,455, nz
Birefringence: Δ = max. 0,028
Crystal class: orthorhombic
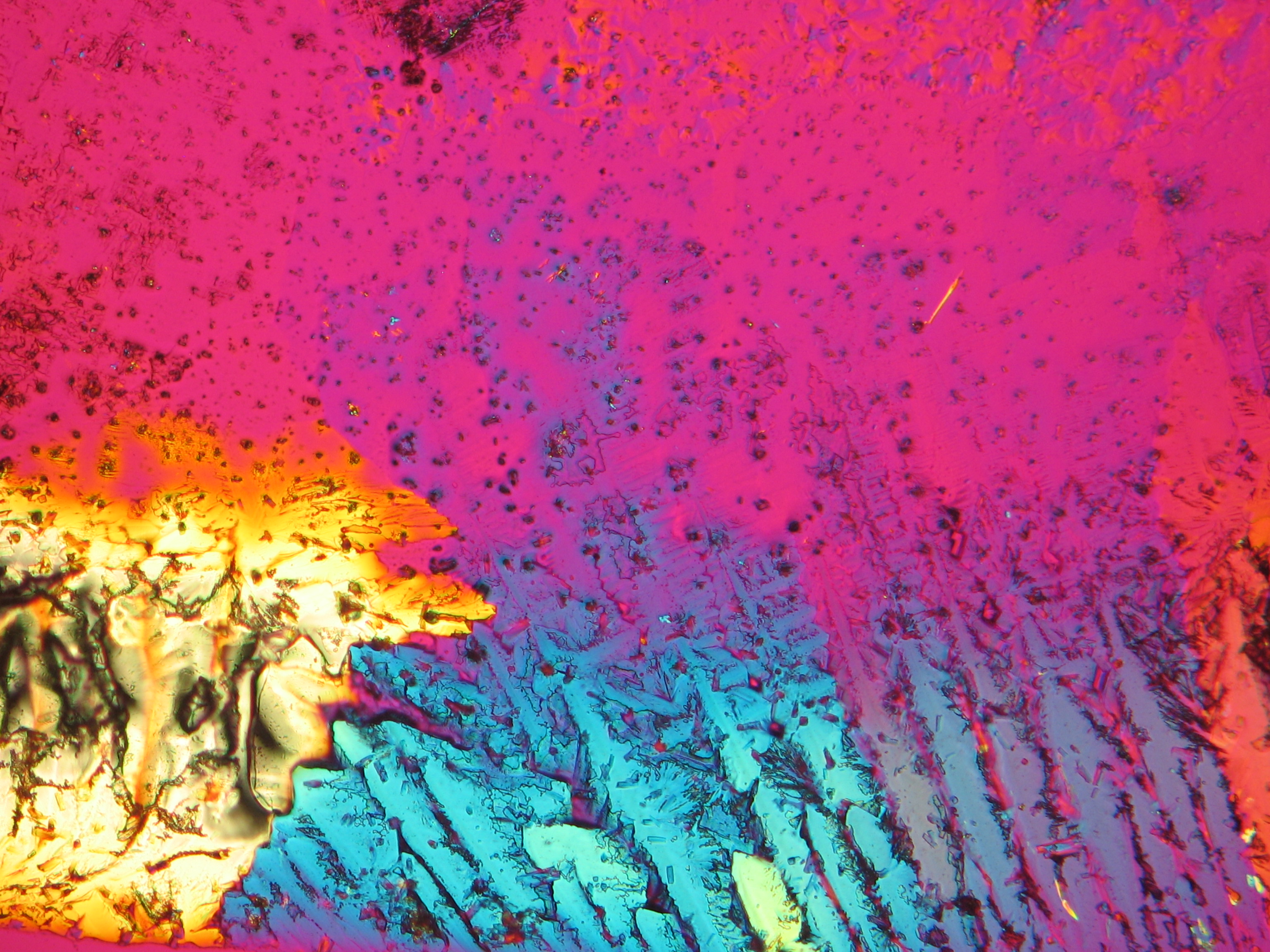
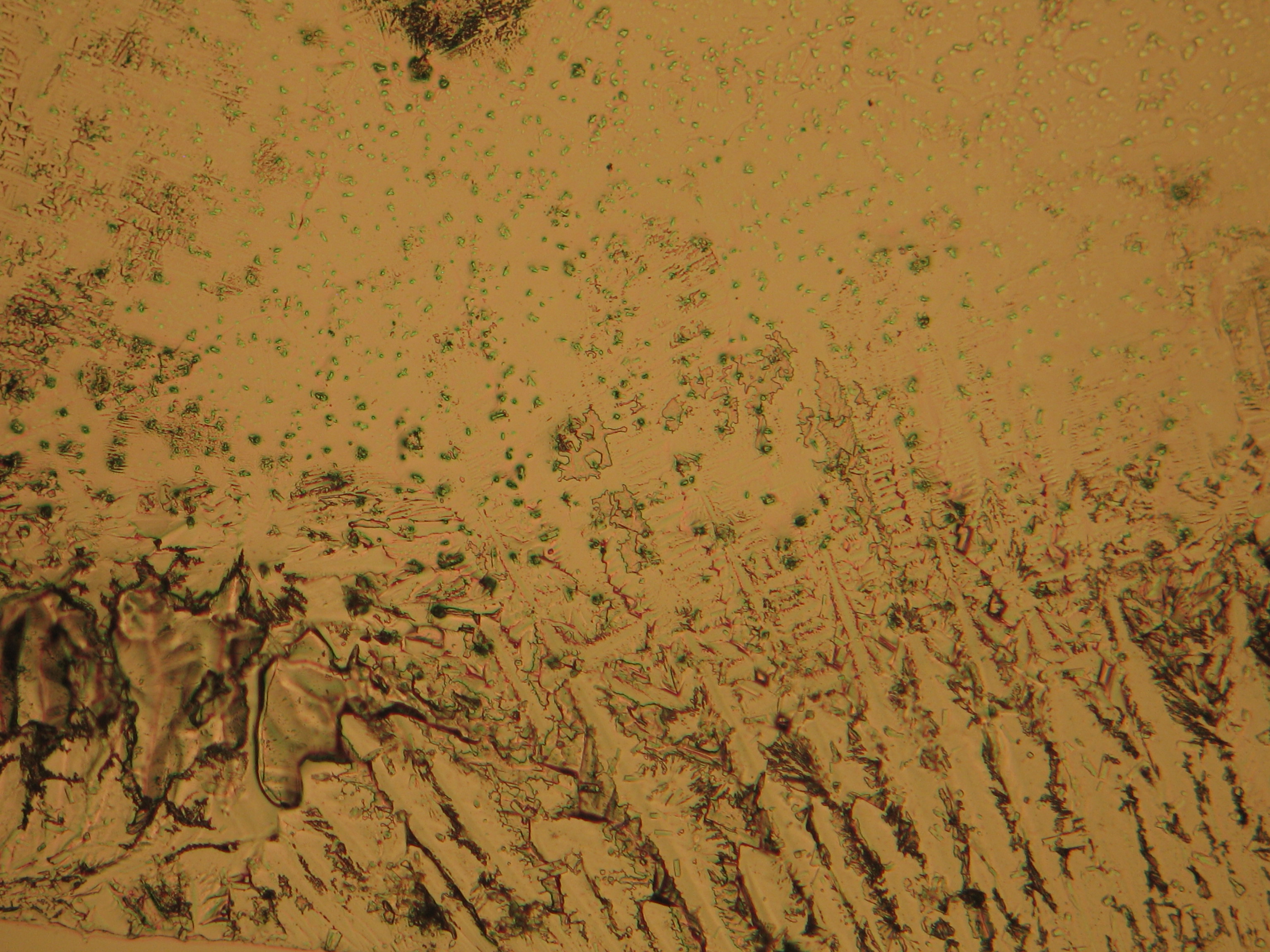
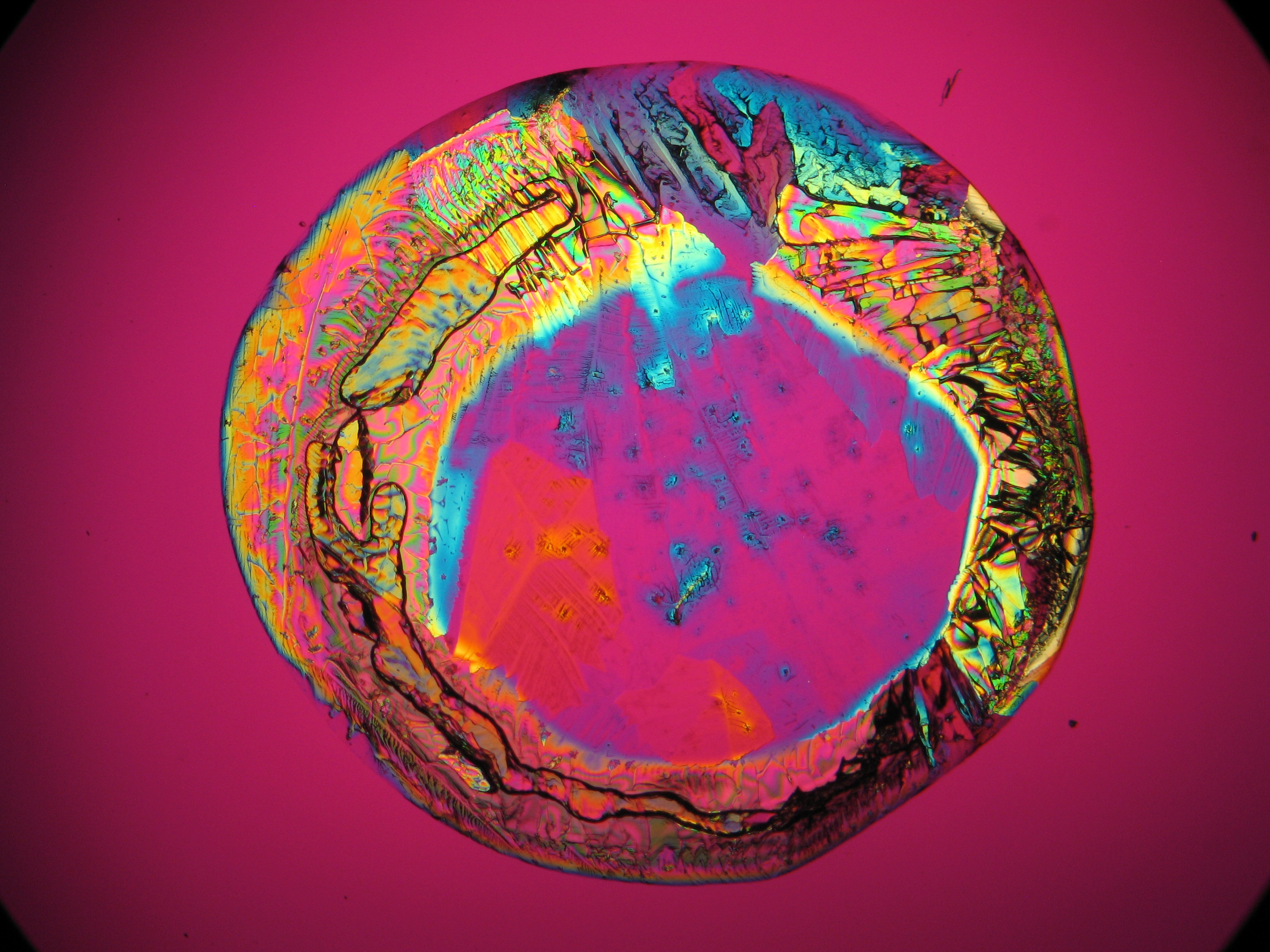
Polarized light microscopic examination:
The allocation of epsomite to the refractive indices is carried out according to the immersion method (successively by means of embedding media nD=1,518; nD=1,47; nD=1,46). When using an immersion medium with a refractive index of nD=1,45 on many single particles a slight but distinct variation in relief is visible at rotation. Because epsomite belongs to the class of orthorhombic crystals, it never appears in an oblique but always in a parallel symmetrical extinction. Beside epsomite, hexahydrite often forms, which is monoclinic and has nearly identical refractive indices. A distinction between these two hydrate stages of magnesium sulfate is only possible through the allocation of the crystal class. Due to the low path difference epsomite crystals usually only show low inference colors in the range of the first order.
Possibility for mistakes:
Epsomite/hexahydrite are to be allocated, once the examination criteria below have been clarified:
- good water solubility
- characteristic appearance at recrystallization
- low birefringence
Salts and deterioration pattern[edit]
On objects[edit]
Under the polarising microscope[edit]
- Crystallized from a water solution on a glass slide
Weblinks[edit]
Literature[edit]
| [Mainusch:2001] | Mainusch, Nils (2001): Erstellung einer Materialsammlung zur qualitativen Bestimmung bauschädlicher Salze für Fachleute der Restaurierung, Diplomarbeit, HAWK Hochschule für angewandte Wissenschaft und Kunst Hildesheim/Holzminden/Göttingen, file:Diplomarbeit Nils Mainusch.pdf |   |
| [Stark.etal:1996] | Stark, Jochen; Stürmer, Sylvia (1996): Bauschädliche Salze, Bauhaus-Univ. Weimar |  |
| [Steiger.etal:2011a] | Steiger, Michael; Linnow, Kirsten; Ehrhardt, Dorothee; Rohde, Mandy (2011): Decomposition reactions of magnesium sulfate hydrates and phase equilibria in the MgSO4-H2O and Na+-Mg2+-Cl--SO42--H2O systems with implications for Mars. In: Geochimica et Cosmochimica Act, 75 (12), 3600-3626, 10.1016/j.gca.2011.03.038, |  |