Mirabilite: Difference between revisions
Jump to navigation
Jump to search
Weblinks
No edit summary |
|||
| Line 177: | Line 177: | ||
=== Under the polarising microscope === | === Under the polarising microscope === | ||
<gallery caption=" | <gallery caption="Sodium sulfate crytallisation between two glas slides" widths="200px" heights="150px" perrow="3"> | ||
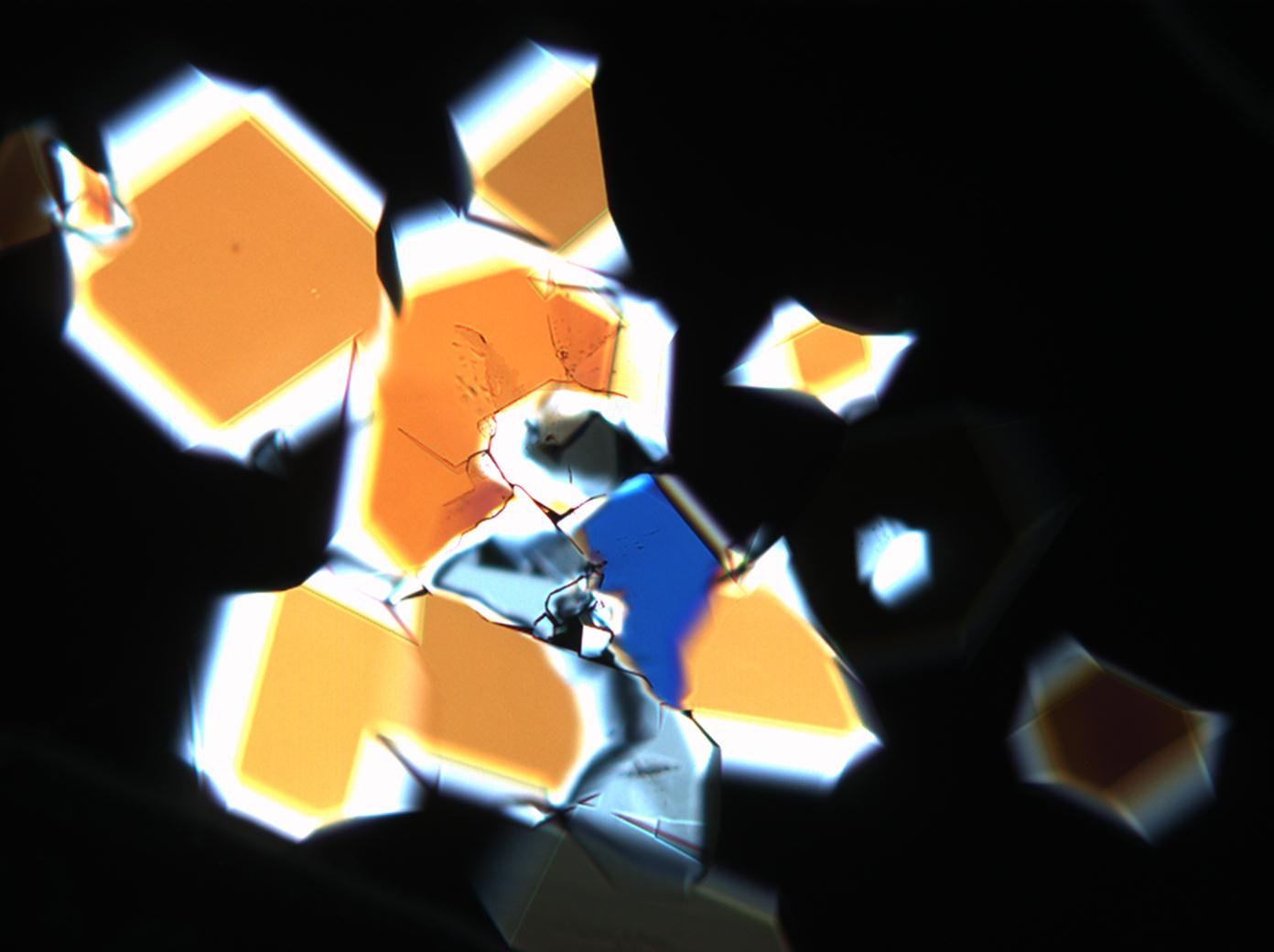
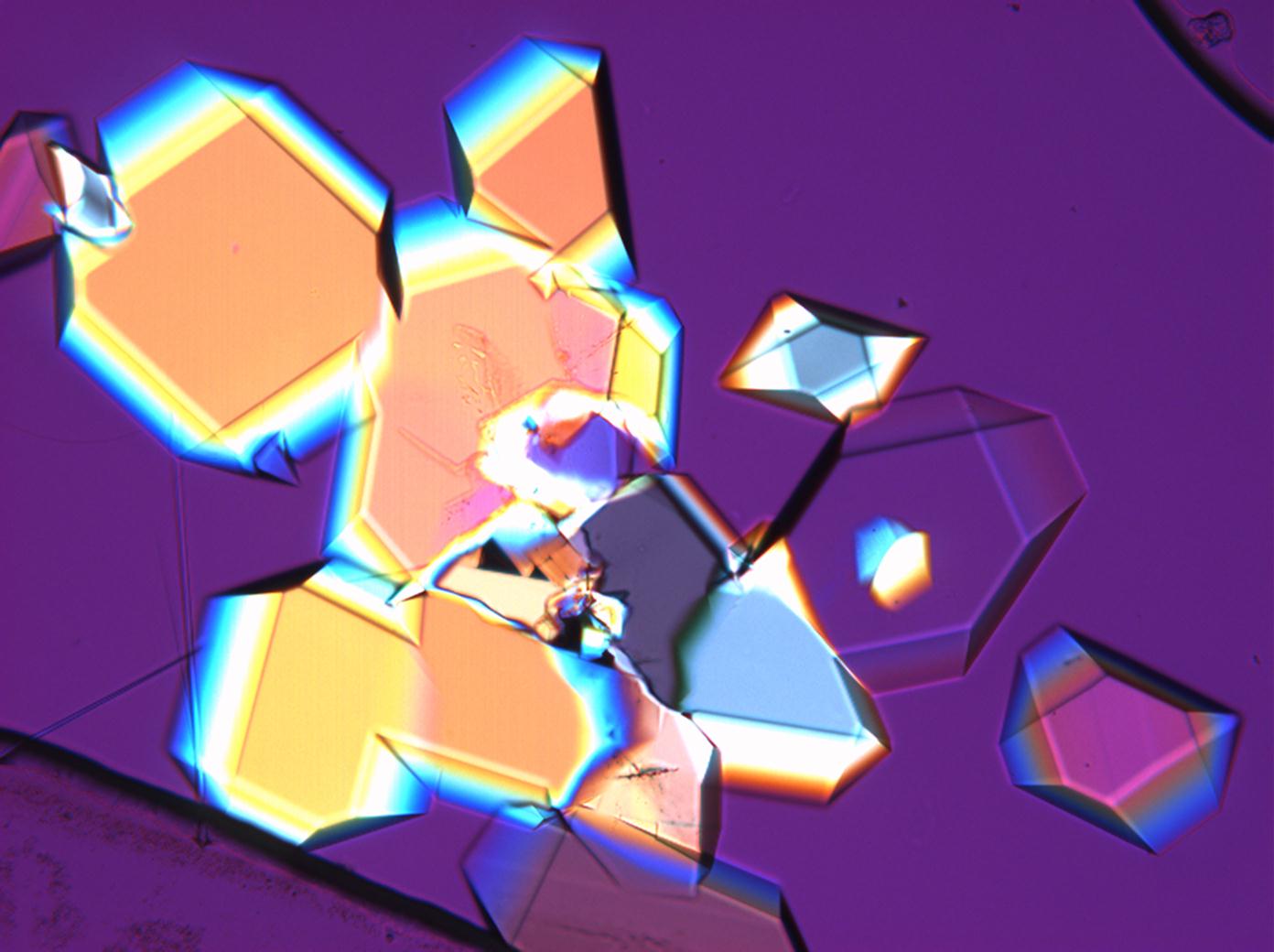
image:HJS_Na2SO4-slides-1-110603.jpg| | image:HJS_Na2SO4-slides-1-110603.jpg|Photo taken with crossed polarisers and red I | ||
image:HJS_Na2SO4-slides-110703-10x-2.jpg| | image:HJS_Na2SO4-slides-110703-10x-2.jpg|Photo taken with crossed polarisers | ||
</gallery> | </gallery> | ||
<!-- | <!-- | ||
| Line 189: | Line 189: | ||
</gallery> | </gallery> | ||
--> | --> | ||
== Weblinks<br> == | == Weblinks<br> == | ||
<references /> | <references /> | ||
Revision as of 16:09, 26 December 2011
<bibimport/>
| Mirabilite[1][2] | |
| |
| Mineralogical name | Mirabilite |
| Chemical name | Sodiumsulfate decahydrate |
| Trivial name | Glauber salt, Reussin, Sulphate of Soda |
| Chemical formula | Na2SO4•10H2O |
| Other forms | Sodiumsulphate heptahydrate Na2SO4•7H2O |
| Crystal system | monoclinic |
| Crystal structure | |
| Deliquescence humidity 20°C | 93.6% (20°C), 90% (23°C), 87% (25°C) |
| Solubility (g/l) at 20°C | 900 g/l |
| Density (g/cm³) | 1.464 g/cm³ |
| Molar volume | 219.8 cm3/mol |
| Molar weight | 322.21 g/mol |
| Transparency | transparent to opaque |
| Cleavage | perfect |
| Crystal habit | |
| Twinning | |
| Phase transition | |
| Chemical behavior | |
| Comments | soluble in water and glycerin, not soluble in pure alcohol easily loses some water, converts to thenardite at 32°C anormal blue or brown interference colours |
| Crystal Optics | |
| Refractive Indices | nx = 1.395 ny = 1.396-1.410 nz = 1.398-1.419 |
| Birefringence | Δ = 0.04-0.023 |
| Optical Orientation | negative |
| Pleochroism | |
| Dispersion | |
| Used Literature | |
| {{{Literature}}} | |
back to Sulfate
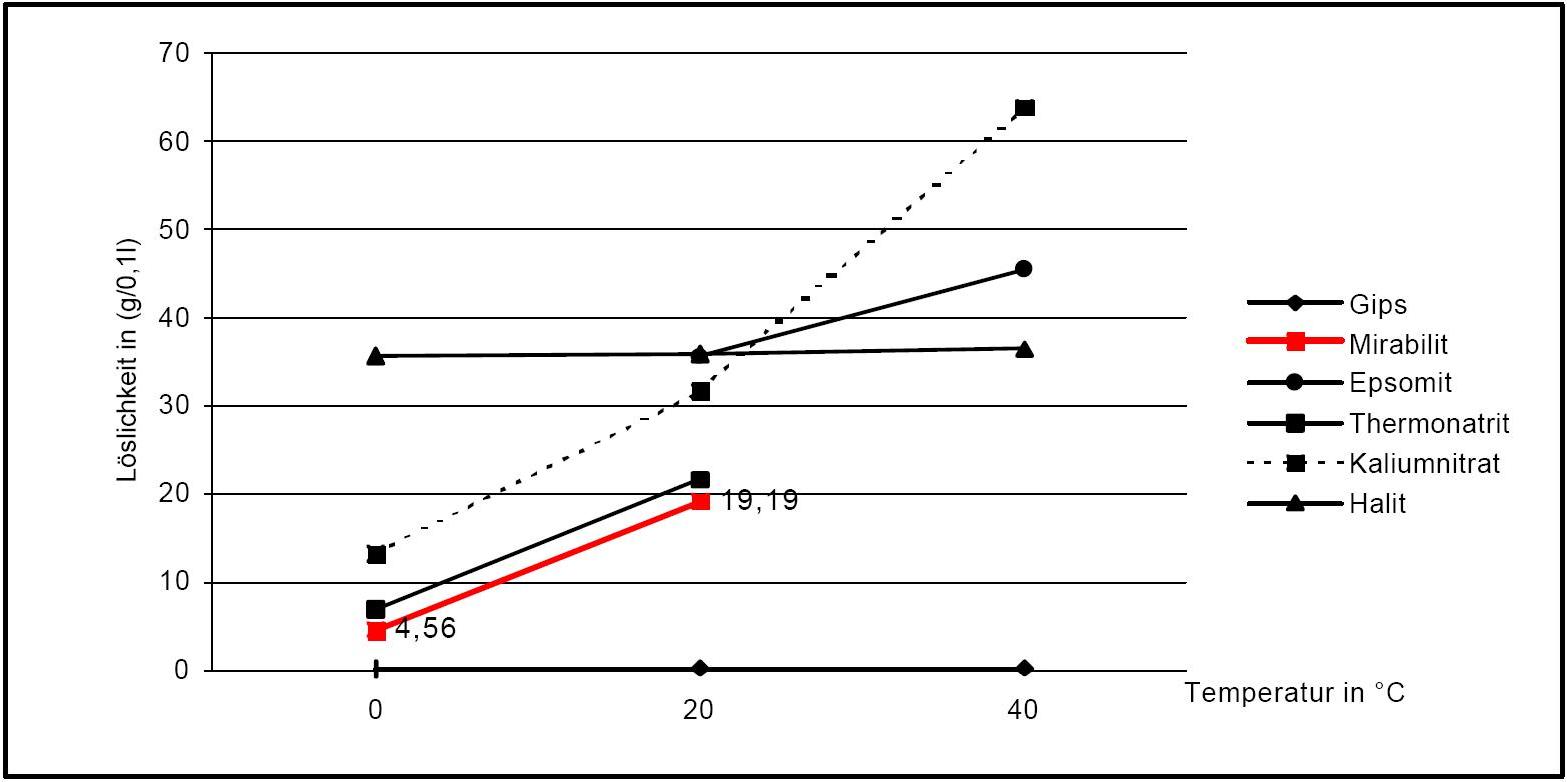
Solubility properties[edit]

Figure1: Solubility of thenardite and mirabilite compared with other salts[after [Stark.etal:1996]Title: Bauschädliche Salze
Author: Stark, Jochen; Stürmer, Sylvia
 ].
].
Author: Stark, Jochen; Stürmer, Sylvia
 ].
].
Under the polarising microscope[edit]
- Sodium sulfate crytallisation between two glas slides
Weblinks
[edit]
- ↑ http://webmineral.com/data/Mirabilite.shtml viewed on 29/07/2010
- ↑ http://www.mindat.org/min-2725.html viewed on 29/07/2010
Literatur[edit]
[Filter missing]