Polarized light microscopy: Difference between revisions
No edit summary |
|||
| Line 28: | Line 28: | ||
<br> '''Advantage:''' | <br> '''Advantage:''' | ||
Polarized light microscopy is a quick and convenient method for the [[Microscopic identification of salts| identification of salts]]. The mineralogy and chemistry of salts is determined. Basic polarizing microscopes are portable and can be used in any location, hence salts | Polarized light microscopy is a quick and convenient method for the [[Microscopic identification of salts| identification of salts]]. The mineralogy and chemistry of salts is determined. Basic polarizing microscopes are portable and can be used in any location, hence ''sensitive'' salts can be identified on site. | ||
<br> '''Disadvantage:''' | <br> '''Disadvantage:''' | ||
Revision as of 10:42, 20 September 2013
Authors: Hans-Jürgen Schwarz, Anika Husen
back to Analysis of Salts
Abstract[edit]
The identification of salts with a polarized light microscope is briefly described and includes the advantages and disadvantages of the method.
Introduction[edit]
Polarized light microscopy [Wuelfert:1999]Title: Der Blick ins Bild
Author: Wülfert, Stefan [McCrone.etal:1984]Title: Polarized light microscopy
[McCrone.etal:1984]Title: Polarized light microscopy
Author: McCrone, W. C.,; McCrone, L. B. ; Delly, J. G. is used especially for the examination of anisotropic (birefringent) objects [1]. This microscope differs from ordinary microscopes by having a precisely centered rotating stage and the polarizing and analyzing filters introduced (below and above the stage, respectively) in the light path so that the object can be observed under plane polarized light or under crossed polarizers. One filter, referred to as the polarizer, is located in the light path beneath the sample slide. Through it the object is illuminated with linear polarized light. The second filter, called the analyzer, is situated in the observation beam path allowing the analysis of the linear polarized light modified by the object. When the polarizer and the analyzer are "crossed" (referred to as crossed polars) it means that there is a 90° difference in the vibration plane of the light allowed through them. When no sample is present, or an isotropic material is in the beam path, no light will come through.
is used especially for the examination of anisotropic (birefringent) objects [1]. This microscope differs from ordinary microscopes by having a precisely centered rotating stage and the polarizing and analyzing filters introduced (below and above the stage, respectively) in the light path so that the object can be observed under plane polarized light or under crossed polarizers. One filter, referred to as the polarizer, is located in the light path beneath the sample slide. Through it the object is illuminated with linear polarized light. The second filter, called the analyzer, is situated in the observation beam path allowing the analysis of the linear polarized light modified by the object. When the polarizer and the analyzer are "crossed" (referred to as crossed polars) it means that there is a 90° difference in the vibration plane of the light allowed through them. When no sample is present, or an isotropic material is in the beam path, no light will come through.
In polarized light microscopy the direct (orthoscopic) or indirect (conoscopic) approach can be applied. The orthoscopic approach is equivalent to ordinary microscopy. When the analyzer is switched on anisotropic bodies appear. Depending on their orientation, thickness and the value of the birefringence[2][3][4] anisotropic bodies appear in the interference color that corresponds to the path difference between the ordinary and extraordinary ray. The light refraction of salt minerals can be estimated relatively easily, when the refraction index of the immersion medium or oil is known.
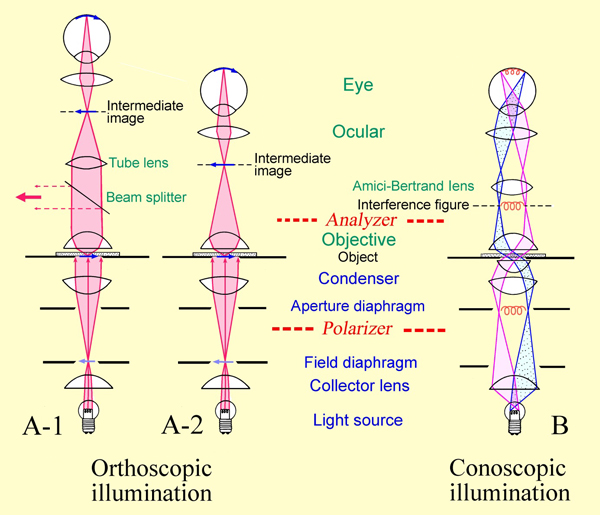
Author: Raith, Michael M.; Raase, Peter; Reinhardt, Jürgen

Conoscopic approach: By switching on an additional lens (Amici-Bertrand lens) or by removal of an eyepiece, the back focal plane of the objective is pictured in the intermediate image plane, seen through the eyepiece. While in the orthoscopic approach every image point corresponds to an object point, in the conoscopic approach every image point corresponds to a parallel beam of light. Therefore the image gives information about the directionality of the birefringence (as far as it can be detected by the aperture). Consequently, this method allows to determine whether a crystal is optically uniaxial or biaxial and whether it is optically positive or negative.
For a detailed description of microscopic mineral analysis see [McCrone.etal:1984]Title: Polarized light microscopy
Author: McCrone, W. C.,; McCrone, L. B. ; Delly, J. G. or [Raith.etal:2012]Title: Guide to Thin Section Microscopy, Second Edition
or [Raith.etal:2012]Title: Guide to Thin Section Microscopy, Second Edition
Author: Raith, Michael M.; Raase, Peter; Reinhardt, Jürgen [6].
[6].
Advantage:
Polarized light microscopy is a quick and convenient method for the identification of salts. The mineralogy and chemistry of salts is determined. Basic polarizing microscopes are portable and can be used in any location, hence sensitive salts can be identified on site.
Disadvantage:
Some salts are difficult to identify. Quantitative identification is not possible.
Weblinks[edit]
- ↑ http://www.microscopy-uk.org.uk/mag/artnov08/rd-crystals.html, gesehen 19.11.2009
- ↑ http://e3.pphysik.uni-dortmund.de/~suter/Vorlesung/Physik_B3_SS03/6.5_Polarisation.pdf, gesehen 19.11.2009
- ↑ http://www.gemmologie.at/mediaCache/Doppelbrechung_270385.pdf, gesehen 19.11.2009
- ↑ http://www.physik.uni-jena.de/inst/iao/applets/doppelbrechung/doppelbrechung.html, gesehen 19.11.2009
- ↑ http://www.igw.uni-jena.de/mineral/downloads/polarisationsmikr.pdf gesehen 16.07.2010
- ↑ http://www.dmg-home.de/pdf/Guide-print%20quality.pdf
Literature[edit]
| [McCrone.etal:1984] | McCrone, W. C.,; McCrone, L. B. ; Delly, J. G. (1984): Polarized light microscopy, McCrone Research Institute, Chicago, 9th ed. 1995 |  |
| [Raith.etal:2012] | Raith, Michael M.; Raase, Peter; Reinhardt, Jürgen (2012): Guide to Thin Section Microscopy, Second Edition, online publication, Url, |  |
| [Wuelfert:1999] | Wülfert, Stefan (1999): Der Blick ins Bild, Ravensburger Buchverlag |  |