Magnesium sulfate: Difference between revisions
(Created page with "Author: Amelie Stahlbuhk <br> back to Sulfate {{UnderConstruction}} ==Abstract== The different hydrated forms of magnesium sulfate and the behaviour co...") |
No edit summary |
||
| Line 79: | Line 79: | ||
In the temperature range of -10 to 100 °C the deliquescence humidities of the present hydrated forms (depending on the temperature) lie always above 80 % r.h., so the salts do not belong to the hygroscopic salts. | In the temperature range of -10 to 100 °C the deliquescence humidities of the present hydrated forms (depending on the temperature) lie always above 80 % r.h., so the salts do not belong to the hygroscopic salts. | ||
<!-- | |||
== Hydration behavior == | |||
The system MgSO<sub>4</sub> – H<sub>2</sub>O: the listed hydrate stages of magnesium sulfates are known to be stable compounds. With the exception of magnesium sulfate- 12- hydrate, all the above stages of the chemically combined water of magnesium sulfate on monuments are visible, but essentially only epsomite, [[hexahydrite]], [[pentahydrite]] and [[kieserite]] occur. | |||
At room temperature and an RH between 50%-90%, epsomite is the most stable hydrate stage. If the RH drops significantly lower than 50% at room temperature, the chemically combined water is released and lower hydrate stages are formed. Hexahydrite (MgSO<sub>4</sub><sub></sub> × 6H<sub>2</sub>O) as pure salt is only stable at a temperature range of approx. 48°C to 67,5 °C. | |||
Pentahydrite has been thought to be metastable or unstable when exposed to the air, nevertheless the existence of these two salt phases has been detected by X-ray diffraction on building structures. The expulsion of the hydrate water can occur up to the formation of kieserite at elevated temperatures. | |||
== Hydration pressure == | |||
Possible variations in magnesium sulfate´s content of chemically combined water on constructions are a fact, and it is likely, that changes in the hydrate stages [[pentahydrite]], [[hexahydrite]] and epsomite (subject to climatic fluctuations) are taking place in situ. The addition of a water molecule into the crystal lattice of hexahydrite, resulting in the transformation to epsomite, is associated with an increase in volume of around 10%. At temperatures from 0-20°C and 70% RH the resulting hydration pressure can be specified at values between 6,8 –9,7 N/mm<sup>2</sup>. The conversion from kieserite to hexahydrite causes an increase in volume of approx. 140% [according to <bib id="Stark.etal:1996"/>]. | |||
== Analytical identification == | |||
On objects, crystallized magnesium sulfate can appear in differing morphologies, but specific forms appear more frequently. One examined object displayed a loose crust of an opaque, gray to yellow substance in situ, which was a mixture of epsomite and hexahydrite (part of the thesis by Mainusch <bib id= "Mainusch:2001"/>). At the monastery church St. Johann at Müstair epsomite appeared as “granular crusts”. Magnesium sulfate efflorescence in the shape of salt whiskers was detected at St. Georgs church in Steiermark (Styria). | |||
<!-- | |||
=== Micro- chemistry === | |||
--> | |||
=== Microscopy === | |||
'''Laboratory examination:''' | |||
Breathing onto a (predominantly) magnesium sulfate efflorescence, causes no observable macroscopic changes. However, characteristics are: the good solubility in water, a pH of 7, the formation of a ring-shaped slightly raised seam (visible by the naked eye), or a transparent layer that remains in place after the evaporation of the aqueous solvent. | |||
In laboratory tests on objects the results should be double checked with the microscope. Observations of the raw sample material and the recrystallized salt, show that magnesium sulfate crystals appear in unspecific forms in sample material. | |||
Experiments to produce well-shaped, single magnesium sulfate crystals, by recrystallization in aqueous solution have not been very successful, because of a strong tendency for intergrowth. Usually the previously mentioned, distinct, ring-shaped seam of intergrown crystals forms, when the magnesium sulfate containing solute is evaporated. Epsomite shows a low solubility in anhydrous ethanol and glycerin under the microscope. | |||
For further examinations using [[polarized light microscopy|polarized light microscopy]], a considerable difficulty is the creation of good preparations, due to the tendency of magnesium sulfate to form only few equidimensional or elongated single crystals. The low solubility in ethanol, nevertheless allows to isolate single particles by repeatedly adding recrystallized material. Both on the base material as well as the recrystallized product, examination using polarized light microscopy should be carried out.<br> | |||
'''Refractive indices''': n<sub>X</sub> = 1,433, n<sub>y</sub> = 1,455, n<sub>z</sub> <br> | |||
'''Birefringence: '''Δ = max. 0,028 <br> | |||
'''Crystal class:''' orthorhombic | |||
<br> | |||
<br> | |||
'''[[Polarized light microscopy|Polarized light microscopic examination:]]'''<br> | |||
The allocation of epsomite to the refractive indices is carried out according to the immersion method (successively by means of embedding media n<sub>D</sub>=1,518; n<sub>D</sub>=1,47; n<sub>D</sub>=1,46). When using an immersion medium with a refractive index of n<sub>D</sub>=1,45 on many single particles a slight but distinct variation in relief is visible at rotation. Because epsomite belongs to the class of orthorhombic crystals, it never appears in an oblique but always in a parallel symmetrical extinction. Beside epsomite, hexahydrite often forms, which is monoclinic and has nearly identical refractive indices. A distinction between these two hydrate stages of magnesium sulfate is only possible through the allocation of the crystal class. Due to the low path difference epsomite crystals usually only show low inference colors in the range of the first order. | |||
'''Possibility for mistakes:'''<br> | |||
Epsomite/[[hexahydrite]] are to be allocated, once the examination criteria below have been clarified: | |||
*good water solubility | |||
*characteristic appearance at recrystallization | |||
*low birefringence | |||
<br> | |||
<!-- | |||
== X- Ray diffraction == | |||
== Raman-Spectroscopiy == | |||
== DTA / TG == | |||
== IR-Spectroscopy == | |||
= <br>Umgang mit Epsomitschäden = | |||
--> | |||
== Salts and deterioration pattern == | |||
=== On objects === | |||
=== Under the polarising microscope === | |||
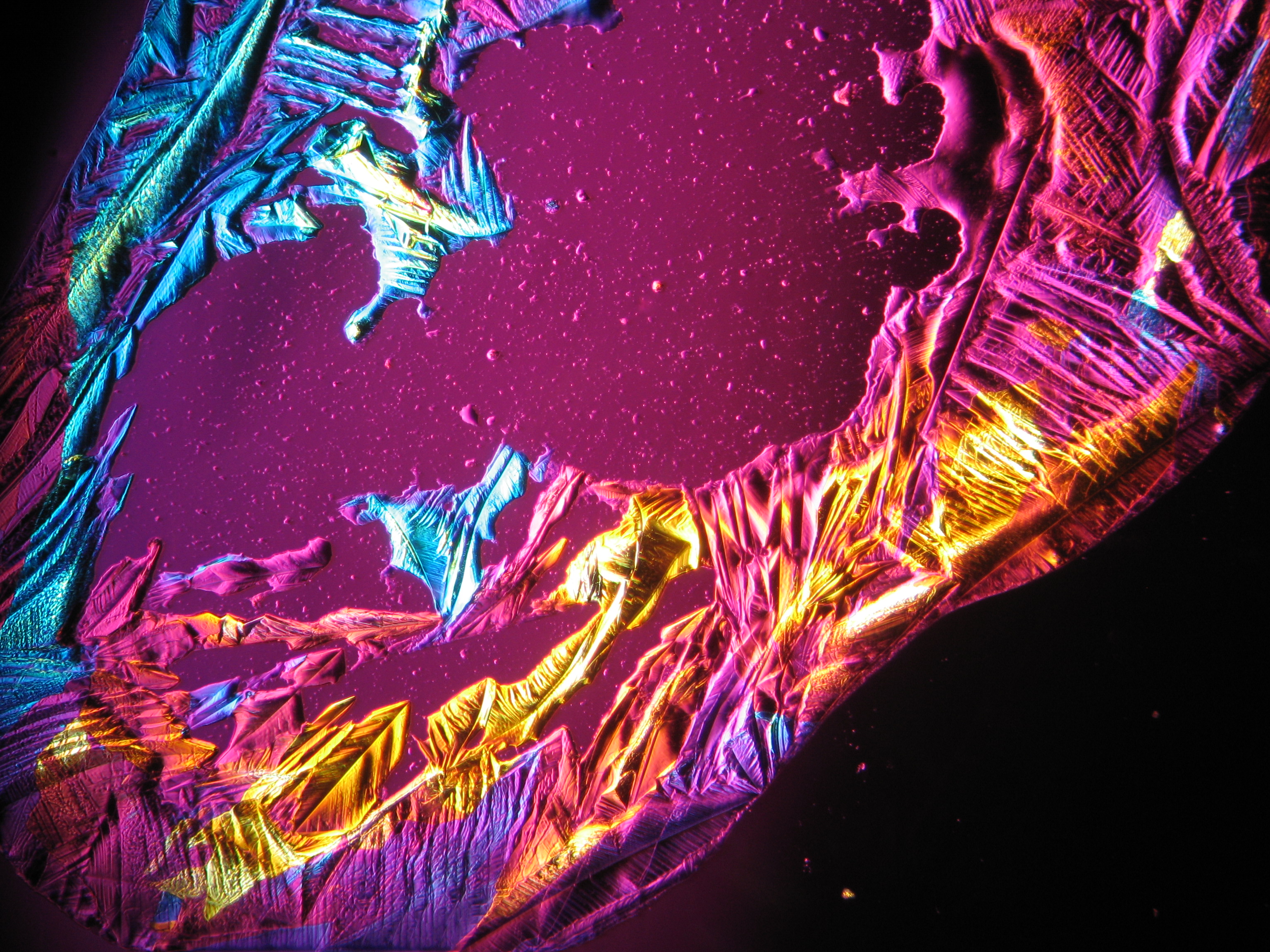
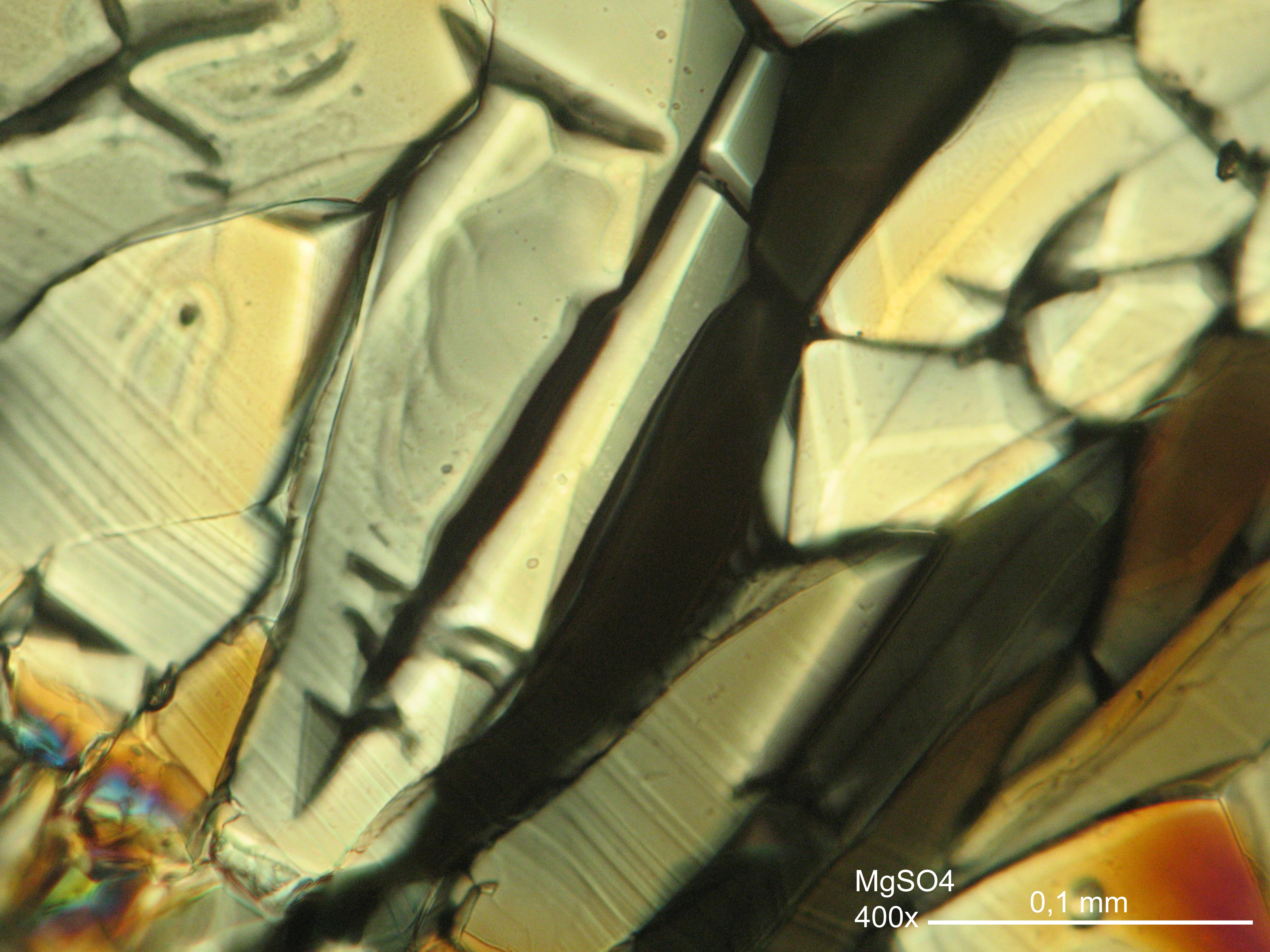
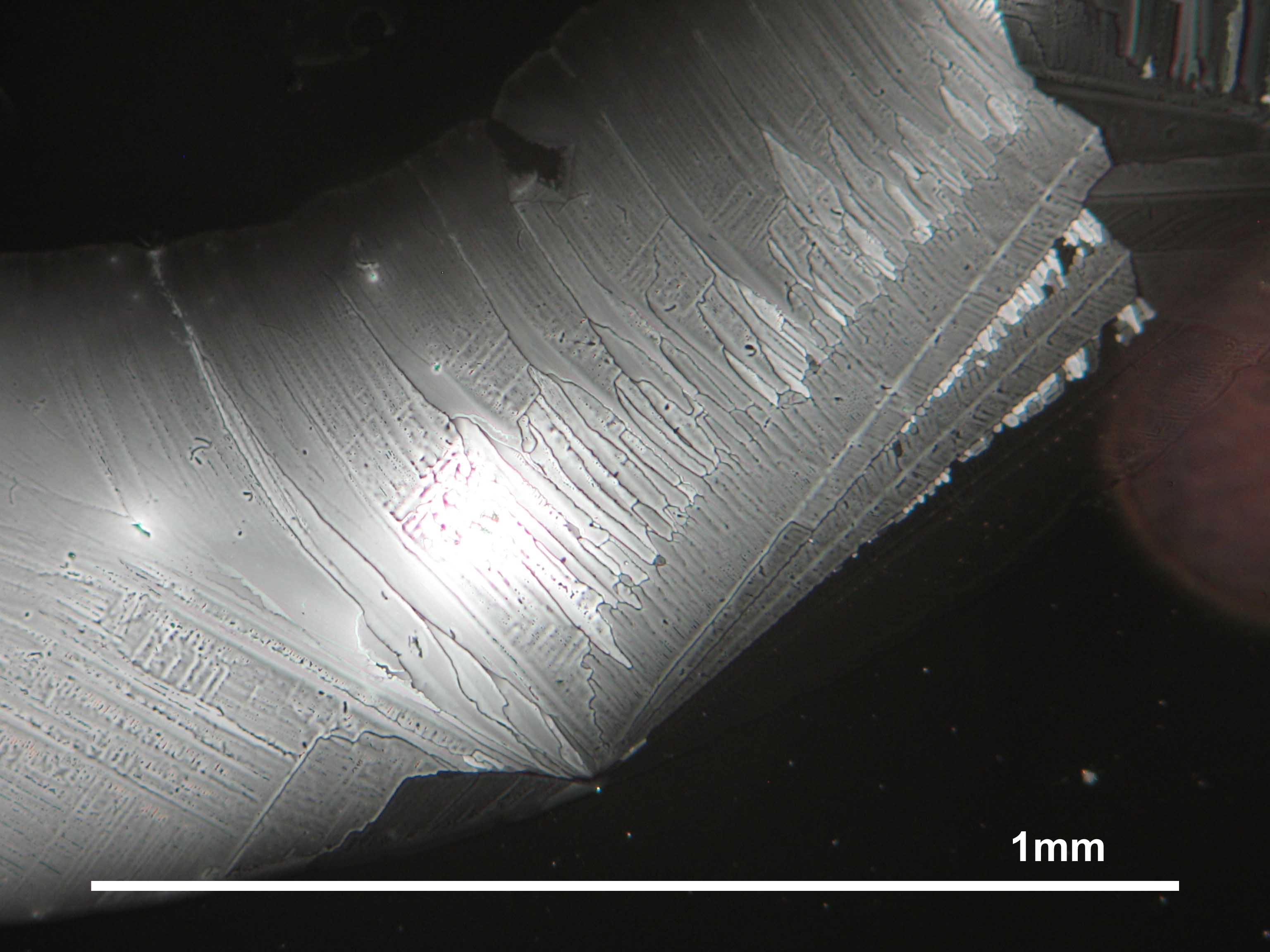
<gallery caption="Crystallized from a water solution on a glass slide " widths="200px" heights="150px" perrow="3"> | |||
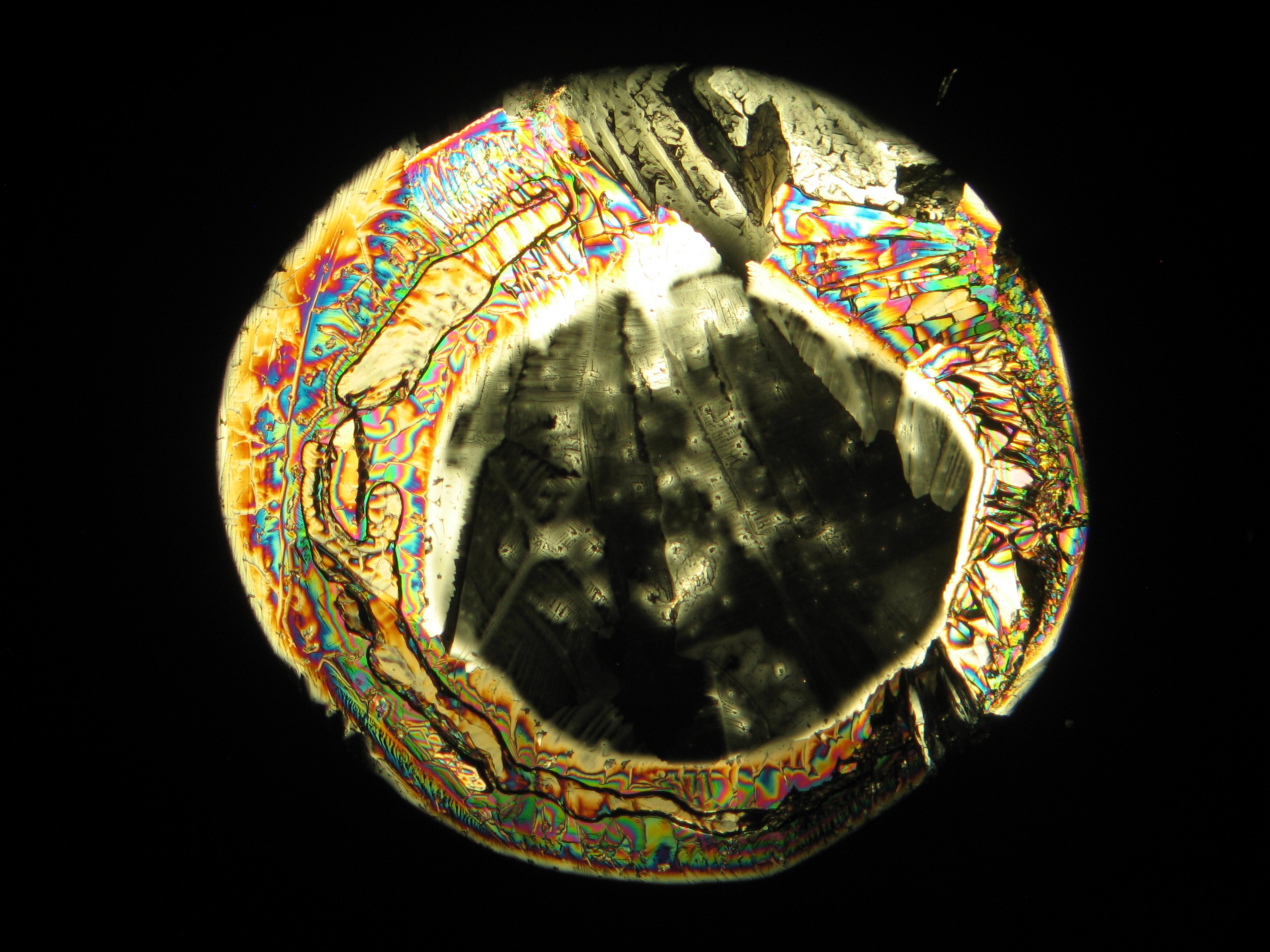
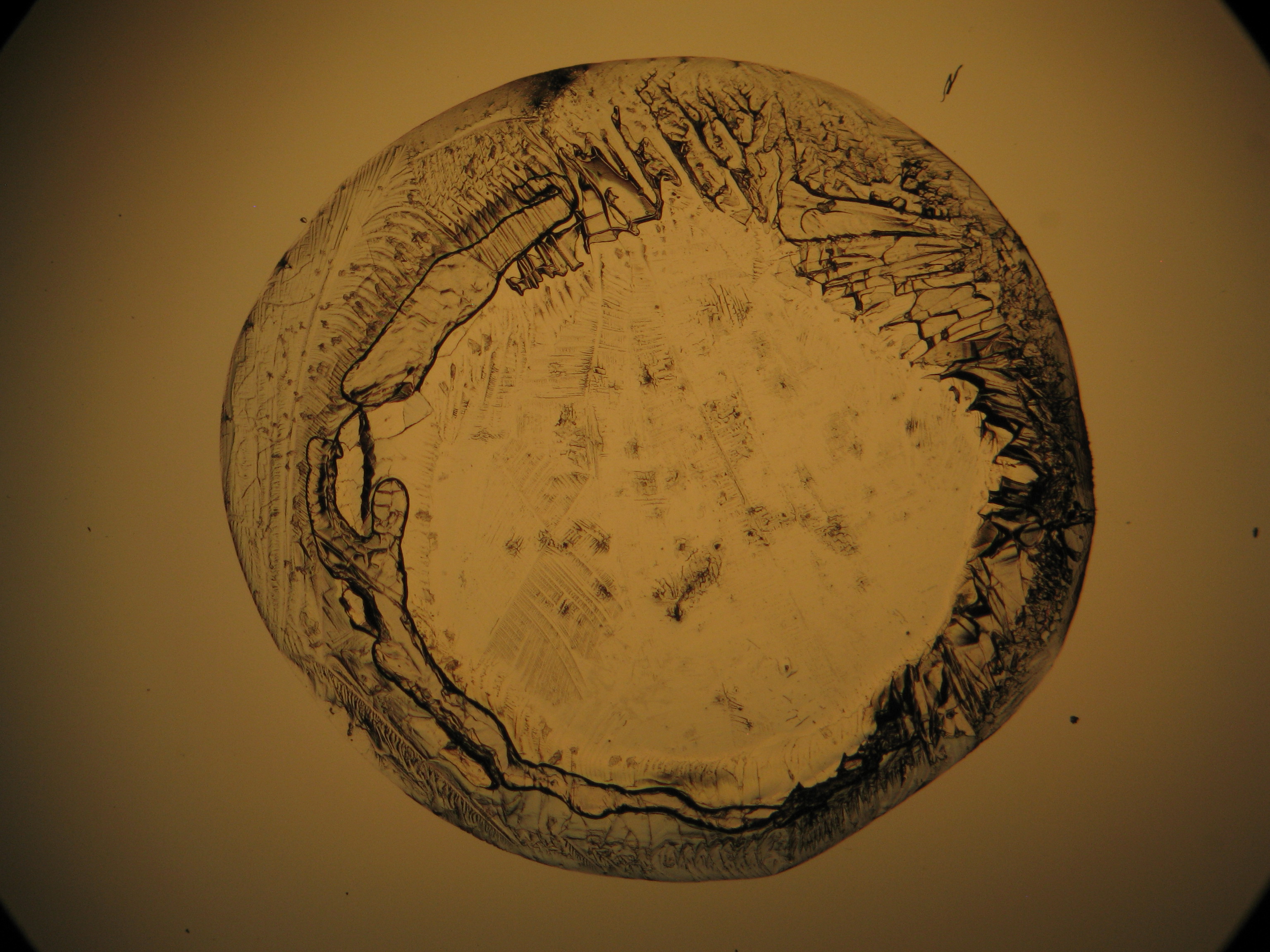
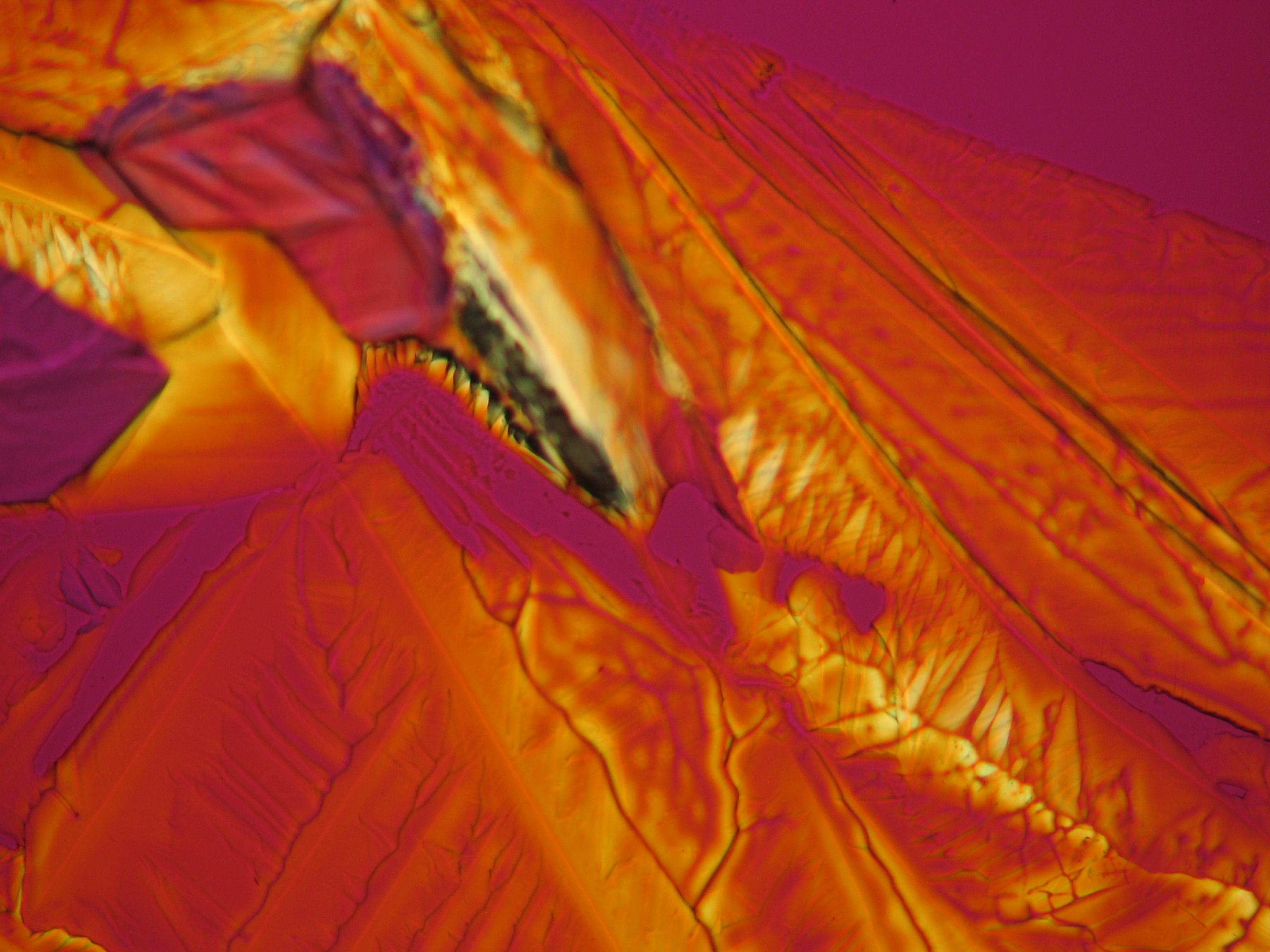
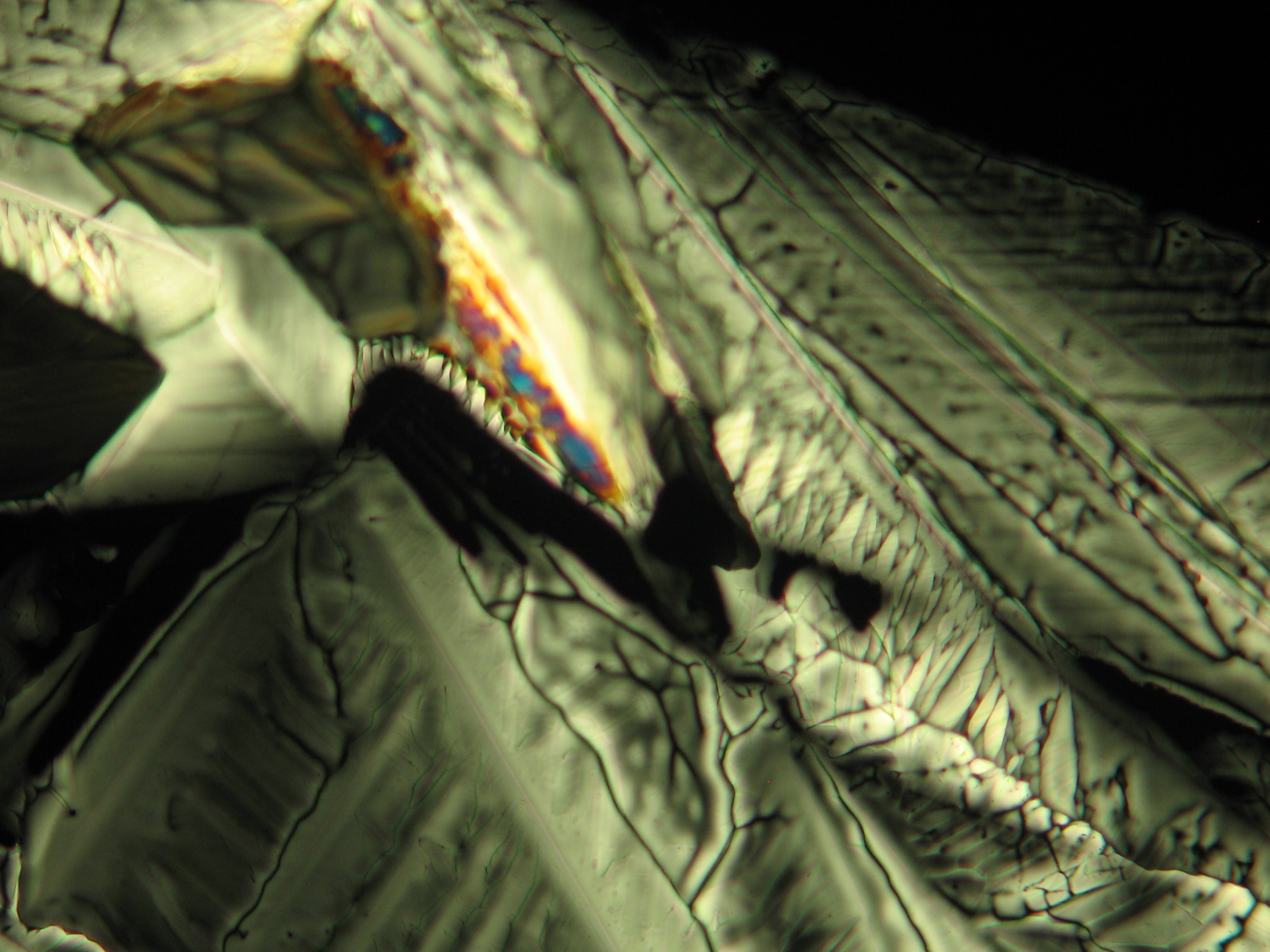
Image:0141 0010 Bb440um 0021.JPG|Sample of a salt crust of magnesium sulfate whiskers | |||
Image:CeS 221209-02-10x-01.JPG|Real sample with magnesium sulfate salts, crystallized from a water solution on a glass slide | |||
Image:CeS 221209-02-10x-02.JPG|Real sample with magnesium sulfate salts, crystallized from a water solution on a glass slide | |||
Image:CeS 221209-04-2,5kx-01.JPG|Real sample with magnesium sulfate salts, crystallized from a water solution on a glass slide | |||
Image:CeS 221209-04-2,5kx-02.JPG|Real sample with magnesium sulfate salts, crystallized from a water solution on a glass slide | |||
Image:CeS 221209-04-2,5kx-03.JPG|Real sample with magnesium sulfate salts, crystallized from a water solution on a glass slide | |||
Image:MGSO4 4.5.2006 (11).JPG|Magnesium- sulfate, crystallized from a water solution on a glass slide | |||
Image:MGSO4 4.5.2006 (12).JPG|Magnesium sulfate, crystallized from a water solution on a glass slide | |||
Image:MGSO4 4.5.2006 (3).JPG|Magnesium sulfate, crystallized from a water solution on a glass slide | |||
Image:Magnesiumsulfat pol 01.jpg|Magnesium sulfate, crystallized from a water solution on a glass slide | |||
Image:MgSO4 pol 400x 01.JPG|Magnesium sulfate, crystallized from a water solution on a glass slide | |||
Image:MgSO4 pol+analy 400x 01.JPG|Magnesium sulfate, crystallized from a water solution on a glass slide | |||
Image:MgSO4 pol+analy+rotII 400x 01.JPG|Magnesium sulfate, crystallized from a water solution on a glass slide | |||
Image:MgSO4 pol+analy.jpg|Magnesium sulfate, crystallized from a water solution on a glass slide | |||
</gallery> | |||
<!-- | |||
== Unter dem Rasterelektronenmikroskop == | |||
--> | |||
==Weblinks== | ==Weblinks== | ||
Revision as of 15:56, 23 March 2015
Author: Amelie Stahlbuhk
back to Sulfate
| This article will be released soon. |
Abstract[edit]
The different hydrated forms of magnesium sulfate and the behaviour concerning solubility and hygroscopicity will be presented.
Hydrated forms[edit]
Kieserite MgSO4•H2O
Sanderite MgSO4•2H2O
Starkeyite MgSO4•4H2O
Pentahydrite MgSO4•5H2O
Hexahydrite MgSO4•6H2O
Epsomite MgSO4•7H2O
Meridianiite MgSO4•11H2O
Solubility[edit]
As it is shown in table 1, the different hydrated forms of magnesium sulfate are easily soluble salts, which leads to a high mobility of the salts in porous materials.
| Hydrated form | Solubility [mol/kg] at 20°C |
| Kieserite | 5.60 |
| Starkeyite | 5.04 |
| Pentahydrite | 4.40 |
| Hexahydrite | 3.61 |
| Epsomite | 2.84 |
Due to the different hydrated forms of magnesium sulfate with stable and meta stable equilibria, the solubility diagram of the system MgSO4-H2O contains more information than diagrams of salts with less or also without any hydrated forms. With the temperature dependence of the solubility it is possible that temperature changes are accompanied by a hydration or a dehydration of a considered phase.
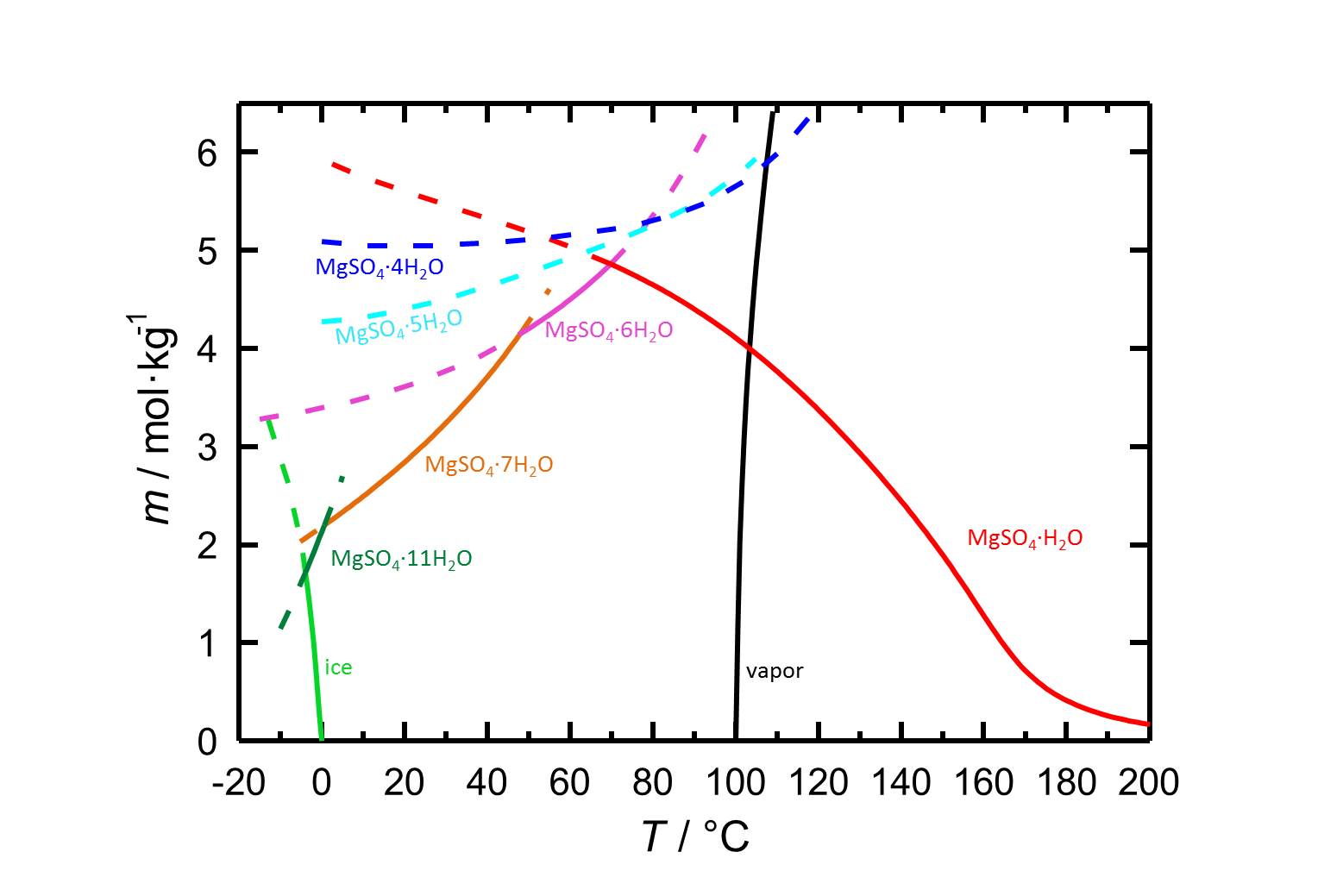
Author: Steiger, Michael; Linnow, Kirsten; Ehrhardt, Dorothee; Rohde, Mandy
 .
.
Hygroscopicity[edit]
In the system MgSO4-H2O changes in temperature or relative humidity may lead to hydration/dehydration or deliquescence/crystallization processes. At 20 °C epsomite is the present crystalline phase when the relative humidity is below its deliquescence humidity of 91.3 %. When the relative humidity reaches values below 47 % the dehydration to lower hydrated levels sets in, as it is represented by the curves of the equilibrium humidities in figure 2.
| Phase transition | Deliquescence or equilibrium humidity at 20°C |
| Epsomite-solution | 91.3 % |
| Epsomite-Hexahydrite | 46.6 % |
| Epsomite-Kieserite | 46.7 % |
| Hexahydrite-Starkeyite | 39.1 % |
In the temperature range of -10 to 100 °C the deliquescence humidities of the present hydrated forms (depending on the temperature) lie always above 80 % r.h., so the salts do not belong to the hygroscopic salts.
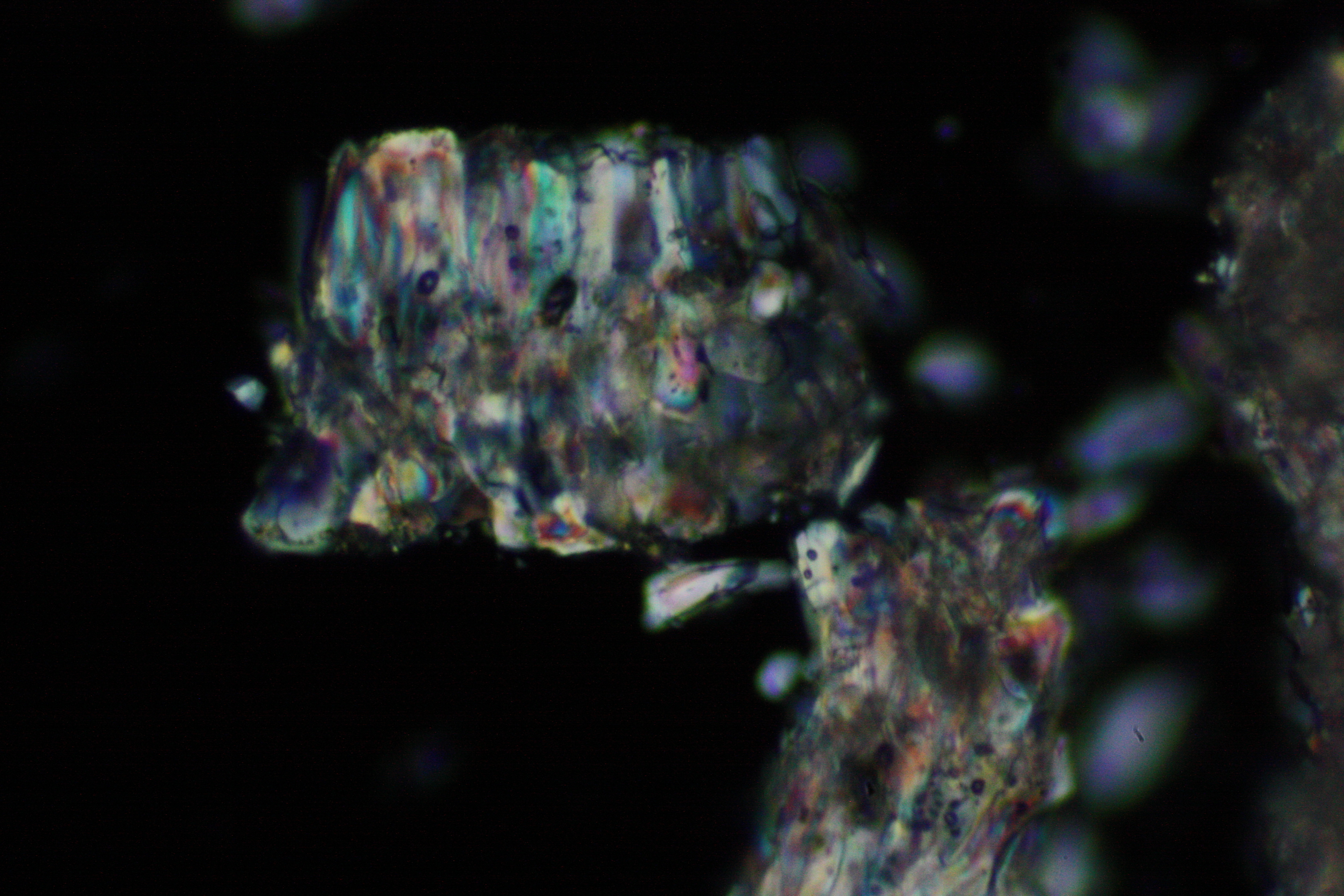
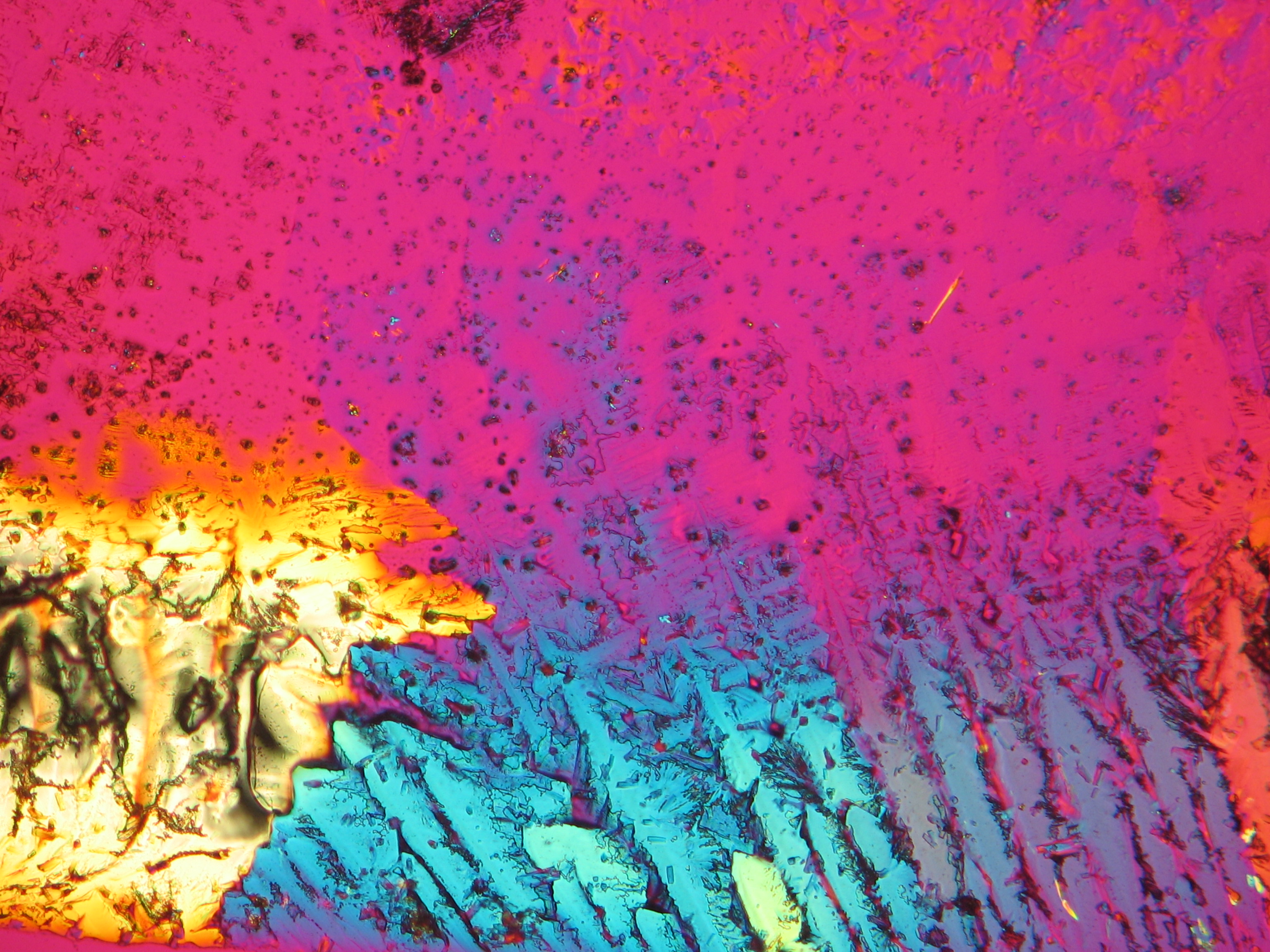
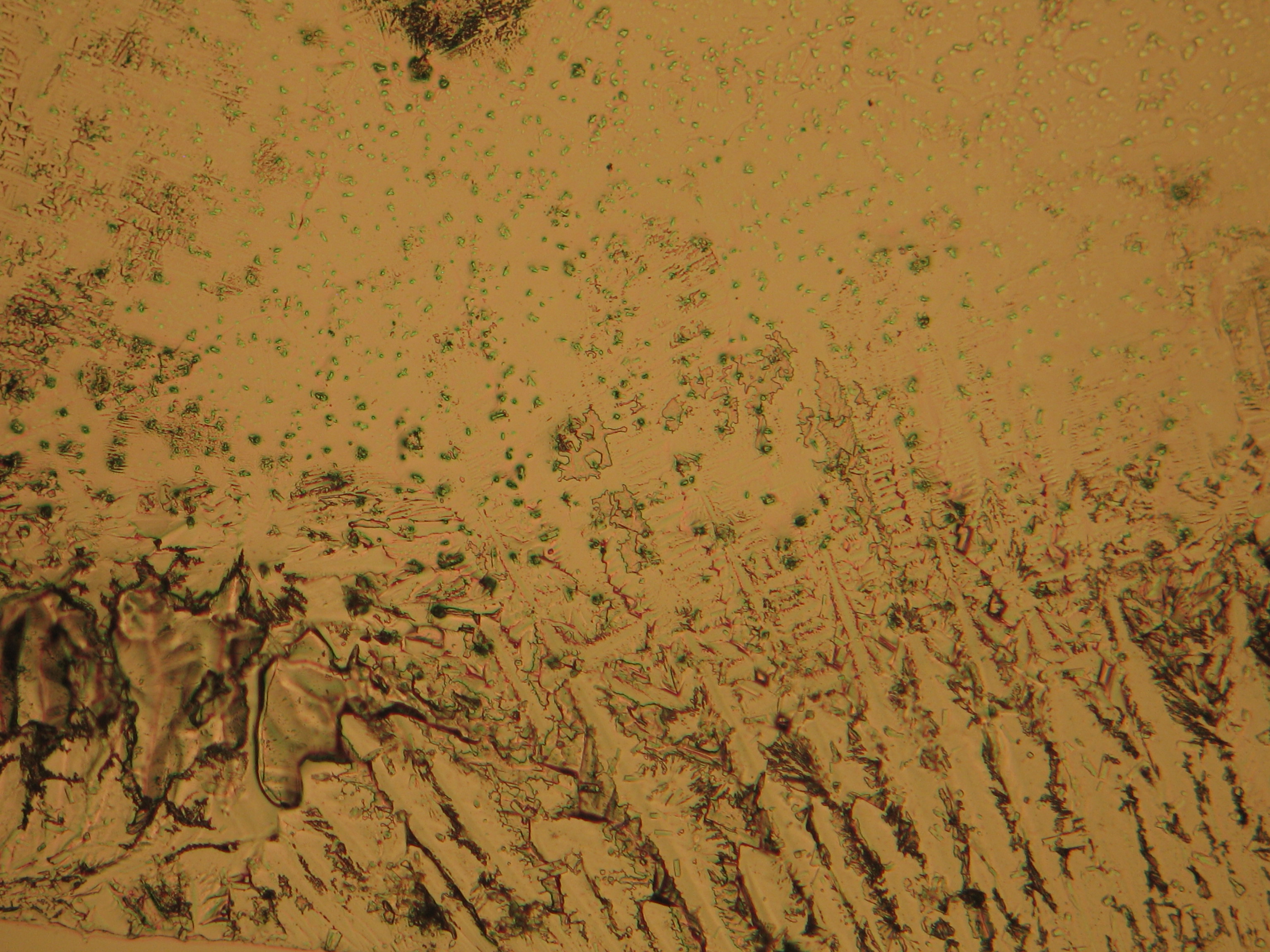
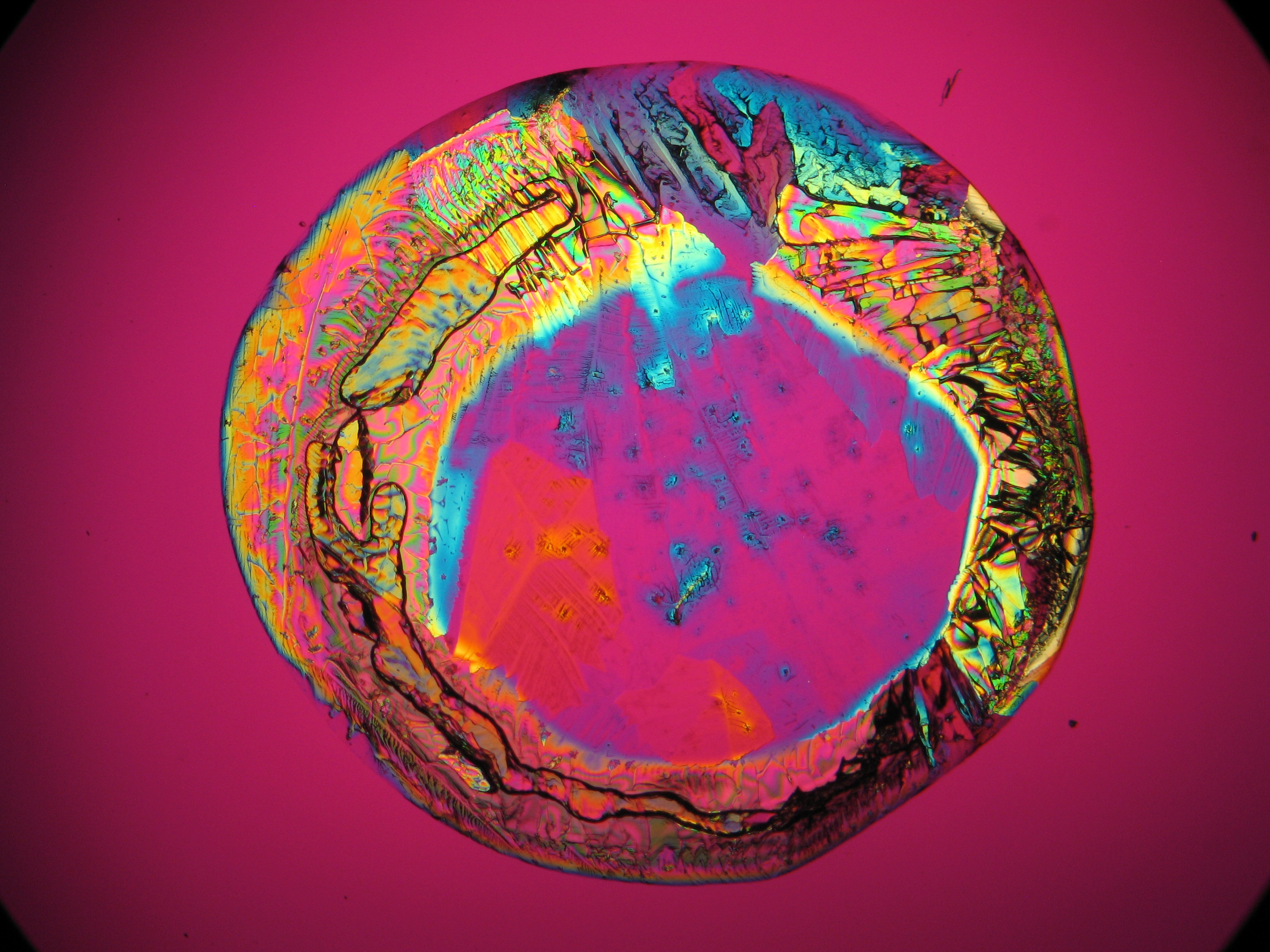
Microscopy[edit]
Laboratory examination:
Breathing onto a (predominantly) magnesium sulfate efflorescence, causes no observable macroscopic changes. However, characteristics are: the good solubility in water, a pH of 7, the formation of a ring-shaped slightly raised seam (visible by the naked eye), or a transparent layer that remains in place after the evaporation of the aqueous solvent.
In laboratory tests on objects the results should be double checked with the microscope. Observations of the raw sample material and the recrystallized salt, show that magnesium sulfate crystals appear in unspecific forms in sample material.
Experiments to produce well-shaped, single magnesium sulfate crystals, by recrystallization in aqueous solution have not been very successful, because of a strong tendency for intergrowth. Usually the previously mentioned, distinct, ring-shaped seam of intergrown crystals forms, when the magnesium sulfate containing solute is evaporated. Epsomite shows a low solubility in anhydrous ethanol and glycerin under the microscope.
For further examinations using polarized light microscopy, a considerable difficulty is the creation of good preparations, due to the tendency of magnesium sulfate to form only few equidimensional or elongated single crystals. The low solubility in ethanol, nevertheless allows to isolate single particles by repeatedly adding recrystallized material. Both on the base material as well as the recrystallized product, examination using polarized light microscopy should be carried out.
Refractive indices: nX = 1,433, ny = 1,455, nz
Birefringence: Δ = max. 0,028
Crystal class: orthorhombic
Polarized light microscopic examination:
The allocation of epsomite to the refractive indices is carried out according to the immersion method (successively by means of embedding media nD=1,518; nD=1,47; nD=1,46). When using an immersion medium with a refractive index of nD=1,45 on many single particles a slight but distinct variation in relief is visible at rotation. Because epsomite belongs to the class of orthorhombic crystals, it never appears in an oblique but always in a parallel symmetrical extinction. Beside epsomite, hexahydrite often forms, which is monoclinic and has nearly identical refractive indices. A distinction between these two hydrate stages of magnesium sulfate is only possible through the allocation of the crystal class. Due to the low path difference epsomite crystals usually only show low inference colors in the range of the first order.
Possibility for mistakes:
Epsomite/hexahydrite are to be allocated, once the examination criteria below have been clarified:
- good water solubility
- characteristic appearance at recrystallization
- low birefringence
Salts and deterioration pattern[edit]
On objects[edit]
Under the polarising microscope[edit]
- Crystallized from a water solution on a glass slide
Weblinks[edit]
Literature[edit]
| [Mainusch:2001] | Mainusch, Nils (2001): Erstellung einer Materialsammlung zur qualitativen Bestimmung bauschädlicher Salze für Fachleute der Restaurierung, Diplomarbeit, HAWK Hochschule für angewandte Wissenschaft und Kunst Hildesheim/Holzminden/Göttingen, file:Diplomarbeit Nils Mainusch.pdf |   |
| [Stark.etal:1996] | Stark, Jochen; Stürmer, Sylvia (1996): Bauschädliche Salze, Bauhaus-Univ. Weimar |  |
| [Steiger.etal:2011a] | Steiger, Michael; Linnow, Kirsten; Ehrhardt, Dorothee; Rohde, Mandy (2011): Decomposition reactions of magnesium sulfate hydrates and phase equilibria in the MgSO4-H2O and Na+-Mg2+-Cl--SO42--H2O systems with implications for Mars. In: Geochimica et Cosmochimica Act, 75 (12), 3600-3626, 10.1016/j.gca.2011.03.038, |  |