Thecotrichite: Difference between revisions
No edit summary |
No edit summary Tag: Reverted |
||
| Line 3: | Line 3: | ||
back to [[Organic Salts]] | back to [[Organic Salts]] | ||
== '''Phase diagram of the quaternary system Ca(CH'''<sub>'''3'''</sub>'''COO)'''<sub>'''2'''</sub>'''–CaCl'''<sub>'''2'''</sub>'''–Ca(NO'''<sub>'''3'''</sub>''')'''<sub>'''2'''</sub>'''–H'''<sub>'''2'''</sub>'''O''' == | == '''Phase diagram of the quaternary system Ca(CH'''<sub>'''3'''</sub>'''COO)'''<sub>'''2'''</sub>'''–CaCl'''<sub>'''2'''</sub>'''–Ca(NO'''<sub>'''3'''</sub>''')'''<sub>'''2'''</sub>'''–H'''<sub>'''2'''</sub>'''O''' == | ||
The isothermal phase diagram of the quaternary system Ca(CH<sub>3</sub>COO)<sub>2</sub>–CaCl<sub>2</sub>–Ca(NO<sub>3</sub>)<sub>2</sub>–H<sub>2</sub>O (25°C) | The isothermal phase diagram of the quaternary system Ca(CH<sub>3</sub>COO)<sub>2</sub>–CaCl<sub>2</sub>–Ca(NO<sub>3</sub>)<sub>2</sub>–H<sub>2</sub>O (25°C) ((bib id="Linnow:2007")) is shown as Jännecke projection for the anhydrous level. All solution compositions are given as '''molar ratios''' of Ca(CH<sub>3</sub>COO)<sub>2</sub>, CaCl<sub>2 </sub>and Ca(NO<sub>3</sub>)<sub>2</sub>. | ||
The corners of the triangle represent the three pure salts, the sides represent the three ternary systems Ca(CH<sub>3</sub>COO)<sub>2</sub>–CaCl<sub>2</sub>–H<sub>2</sub>O, Ca(CH<sub>3</sub>COO)<sub>2</sub>–Ca(NO<sub>3</sub>)<sub>2</sub>–H<sub>2</sub>O and CaCl<sub>2</sub>–Ca(NO<sub>3</sub>)<sub>2</sub>–H<sub>2</sub> while the inner surface represents all solution compositions where the three salts are involved. | The corners of the triangle represent the three pure salts, the sides represent the three ternary systems Ca(CH<sub>3</sub>COO)<sub>2</sub>–CaCl<sub>2</sub>–H<sub>2</sub>O, Ca(CH<sub>3</sub>COO)<sub>2</sub>–Ca(NO<sub>3</sub>)<sub>2</sub>–H<sub>2</sub>O and CaCl<sub>2</sub>–Ca(NO<sub>3</sub>)<sub>2</sub>–H<sub>2</sub> while the inner surface represents all solution compositions where the three salts are involved. | ||
| Line 115: | Line 87: | ||
== Literature == | == Literature == | ||
((biblist)) | |||
[[Category:Thecotrichite]][[Category:Acetate]][[Category:Salt]][[Category:approved]][[category:Organic_Salts]][[Category:Linnow,Kirsten]] [[Category:R-MSteiger]][[category:List]] | [[Category:Thecotrichite]][[Category:Acetate]][[Category:Salt]][[Category:approved]][[category:Organic_Salts]][[Category:Linnow,Kirsten]] [[Category:R-MSteiger]][[category:List]] | ||
Revision as of 08:04, 8 August 2023
Author: Kirsten Linnow
back to Organic Salts
Phase diagram of the quaternary system Ca(CH3COO)2–CaCl2–Ca(NO3)2–H2O[edit]
The isothermal phase diagram of the quaternary system Ca(CH3COO)2–CaCl2–Ca(NO3)2–H2O (25°C) ((bib id="Linnow:2007")) is shown as Jännecke projection for the anhydrous level. All solution compositions are given as molar ratios of Ca(CH3COO)2, CaCl2 and Ca(NO3)2. The corners of the triangle represent the three pure salts, the sides represent the three ternary systems Ca(CH3COO)2–CaCl2–H2O, Ca(CH3COO)2–Ca(NO3)2–H2O and CaCl2–Ca(NO3)2–H2 while the inner surface represents all solution compositions where the three salts are involved.
The inner lines delimit the stability fields of a given salt phase. The stability fields represent all solution compositions, which are saturated with respect to only a single salt phase. The univariant lines represent the solution compositions where two salt phases are in equilibrium with the solution.
According to Gibbs’ phase rule in the quaternary system a maximum of three different salt phases can coexist in solution equilibrium. It follows that for each possible combination of three salt phases in the solid phase, only one saturated solution composition is possible. These solution combinations are represented by intersections of the univariant lines.
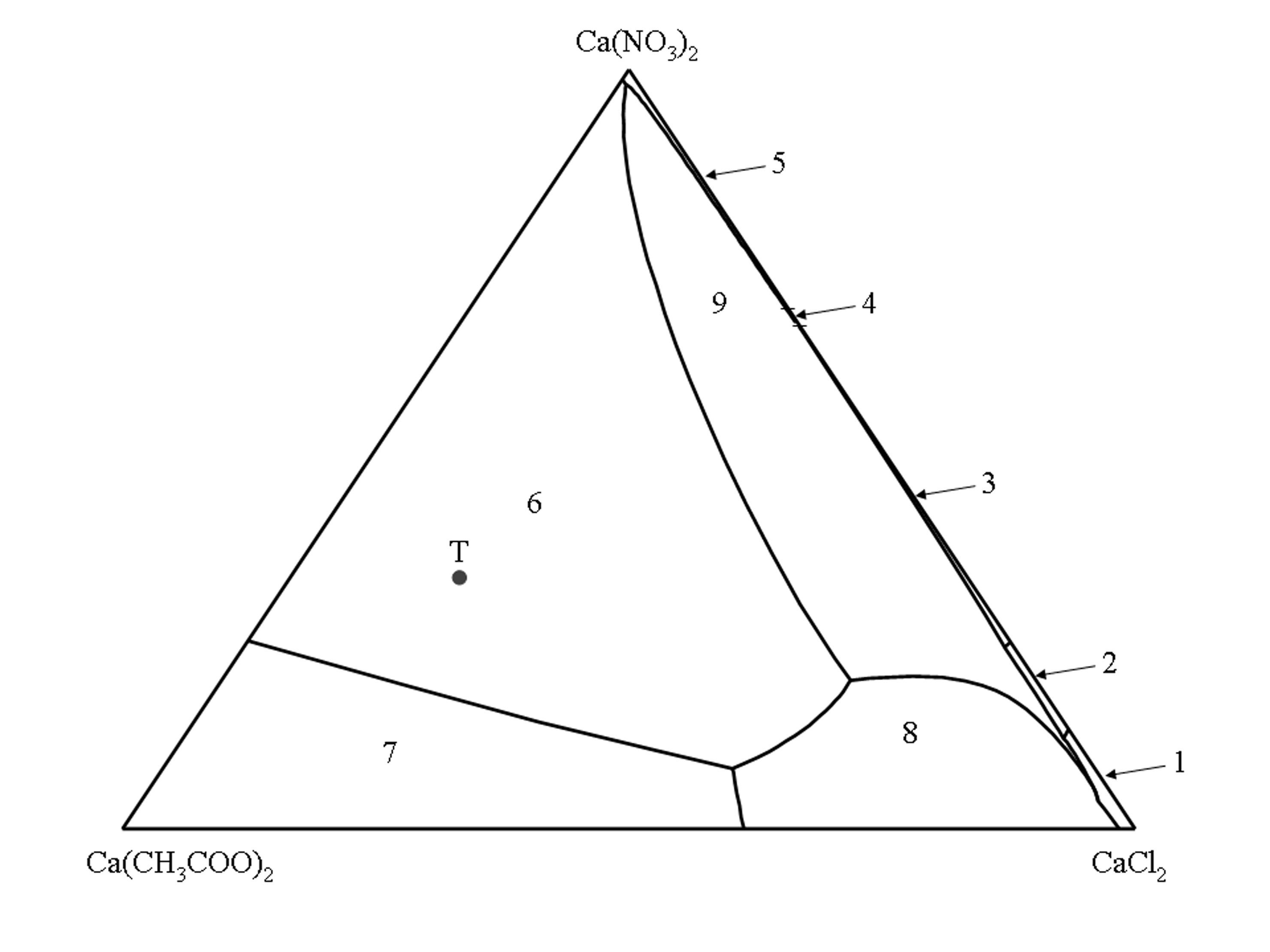
(1) CaCl2 • 6H2O, (2) CaCl2 • 4H2O, (3) CaCl(NO3) • 2H2O, (4) Ca(NO3)2 • 3H2O, (5) Ca(NO3)2 • 4H2O, (6) Ca2(CH3COO)3(NO3) • 2H2O, (7) Ca(CH3COO)2 • H2O, (8) Ca(CH3COO)Cl • 5H2O, (9) Ca3(CH3COO)3Cl(NO3)2 • 7H2O.
Point T in the phase diagram represents the composition of the triple salt thecotrichite (Ca3(CH3COO)3Cl(NO3)2 • 7H2O).
Weblinks[edit]
Literature[edit]
((biblist))