Talk:Chemical methods: Difference between revisions
SLeithaeuser (talk | contribs) No edit summary |
SLeithaeuser (talk | contribs) |
||
| (2 intermediate revisions by the same user not shown) | |||
| Line 26: | Line 26: | ||
== [[ Karl Fischer titration]] == | == [[ Karl Fischer titration]] == | ||
The '''Karl Fischer titration''' | The '''Karl Fischer titration''' is a reaction between the Karl Fischer solution (J<sub>2</sub> und SO<sub>2</sub>) and the sample (contains H<sub>2</sub>O). The following equation applies: | ||
<math>J_2 + SO_2 + 2 H_2O \rightarrow H_2SO_4 + 2 HJ</math> | <math>J_2 + SO_2 + 2 H_2O \rightarrow H_2SO_4 + 2 HJ</math> | ||
The brown iodine water is discolored. When the end of the titration is reached, the sample takes on the brown color (free J<sub>2</sub> is present again), making a visual determination of the point in time possible. The moisture content of the sample is determined | The brown iodine water is discolored. When the end of the titration is reached, the sample takes on the brown color (free J<sub>2</sub> is present again), making a visual determination of the point in time possible. The moisture content of the sample is determined by the amount of Karl Fischer solution needed. With this method even very small traces of water can be detected. However, the crushing of the sample can lead to a measurement error (similar to the carbide method). | ||
== Weblinks == | == Weblinks == | ||
Latest revision as of 23:31, 1 February 2013
Author: Hans-Jürgen Schwarz
back to Moisture
Abstract
This article contains an explanation of the calcium carbide method and the Karl-Fischer method [Kupfer:1997]Title: Materialfeuchtemessung: Grundlagen, Messverfahren, Applikationen, Normen . Both investigation methods require sampling of a building material. The sample size is based on the estimated amount of water in the sample.
. Both investigation methods require sampling of a building material. The sample size is based on the estimated amount of water in the sample.
Calcium carbide method
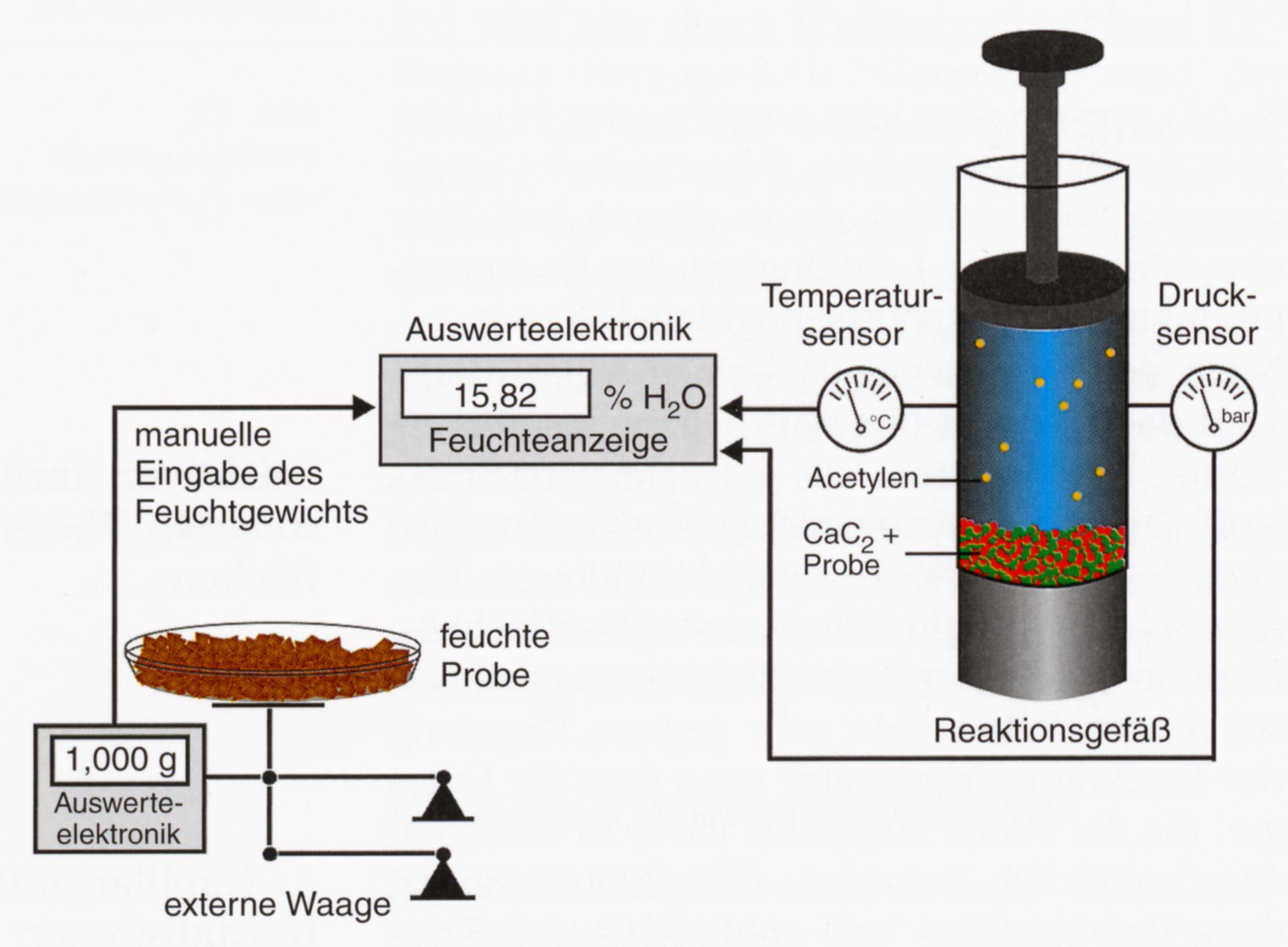
When using the calcium carbide-method[1], a crushed sample has to be introduced into the CM- device. The CM-device is a pressure vessel, which contains a steel sphere. The vial with the sample material in it has to be broken by vigorously shaking the device, the sample material is then mixed with calcium carbide, and the following reaction will take place:
The water contained in the sample reacts with the calcium carbide, producing calcium hydroxide and acetylene. The amount of gas inside the container is a measure for the amount of water contained in the sample. The pressure increase can be read on a built-in pressure gage. Data from tables, which are provided by the supplier, are used for the interpretation of the results. Choosing a sample of an appropriate size requires some experience, because it depends on the moisture content of the sample. The lower the moisture content of a sample, the more material is needed, in order to prevent measurement errors. Also during the crushing of the sample a measurement errors occurs, that results in disparities of 1-3%. The measuring values of this method are often too low, if the sample was not crushed finely enough to cause a reaction that includes all the moisture from the capillary bonds.
Karl Fischer titration
The Karl Fischer titration is a reaction between the Karl Fischer solution (J2 und SO2) and the sample (contains H2O). The following equation applies:
The brown iodine water is discolored. When the end of the titration is reached, the sample takes on the brown color (free J2 is present again), making a visual determination of the point in time possible. The moisture content of the sample is determined by the amount of Karl Fischer solution needed. With this method even very small traces of water can be detected. However, the crushing of the sample can lead to a measurement error (similar to the carbide method).
Weblinks
- ↑ http://de.wikipedia.org/wiki/Calciumcarbid-Verfahren, gesehen am 20.7.2010
Literature
| [Kupfer:1997] | (1997): Materialfeuchtemessung: Grundlagen, Messverfahren, Applikationen, Normen, Expert Verlag |  |