Mirabilite: Difference between revisions
Jump to navigation
Jump to search
No edit summary |
No edit summary |
||
| Line 9: | Line 9: | ||
|Crystal_System = monoclinic | |Crystal_System = monoclinic | ||
|Crystal_Structure = | |Crystal_Structure = | ||
|Deliqueszenzhumidity = 93,6 | |Deliqueszenzhumidity = 93,6% (20°C), 90% (23°C), 87% (25°C) | ||
|Solubility = | |Solubility = 900 g/l | ||
|Density = 1,464 g/cm³ | |Density = 1,464 g/cm³ | ||
|MolVolume = 219,8 cm<sup>3</sup>/mol | |MolVolume = 219,8 cm<sup>3</sup>/mol | ||
| Line 25: | Line 25: | ||
|Phase_Transition = | |Phase_Transition = | ||
|chemBehavior = | |chemBehavior = | ||
|Comments = soluble in water and glycerin,<br> not soluble in pure alcohol | |Comments = soluble in water and glycerin,<br> not soluble in pure alcohol<br> easily loses some water, converts to thenardite at 32°C<br>anormal blue or brown interference colours | ||
}} | }} | ||
Revision as of 09:42, 7 July 2011
| Mirabilite[1][2] | |
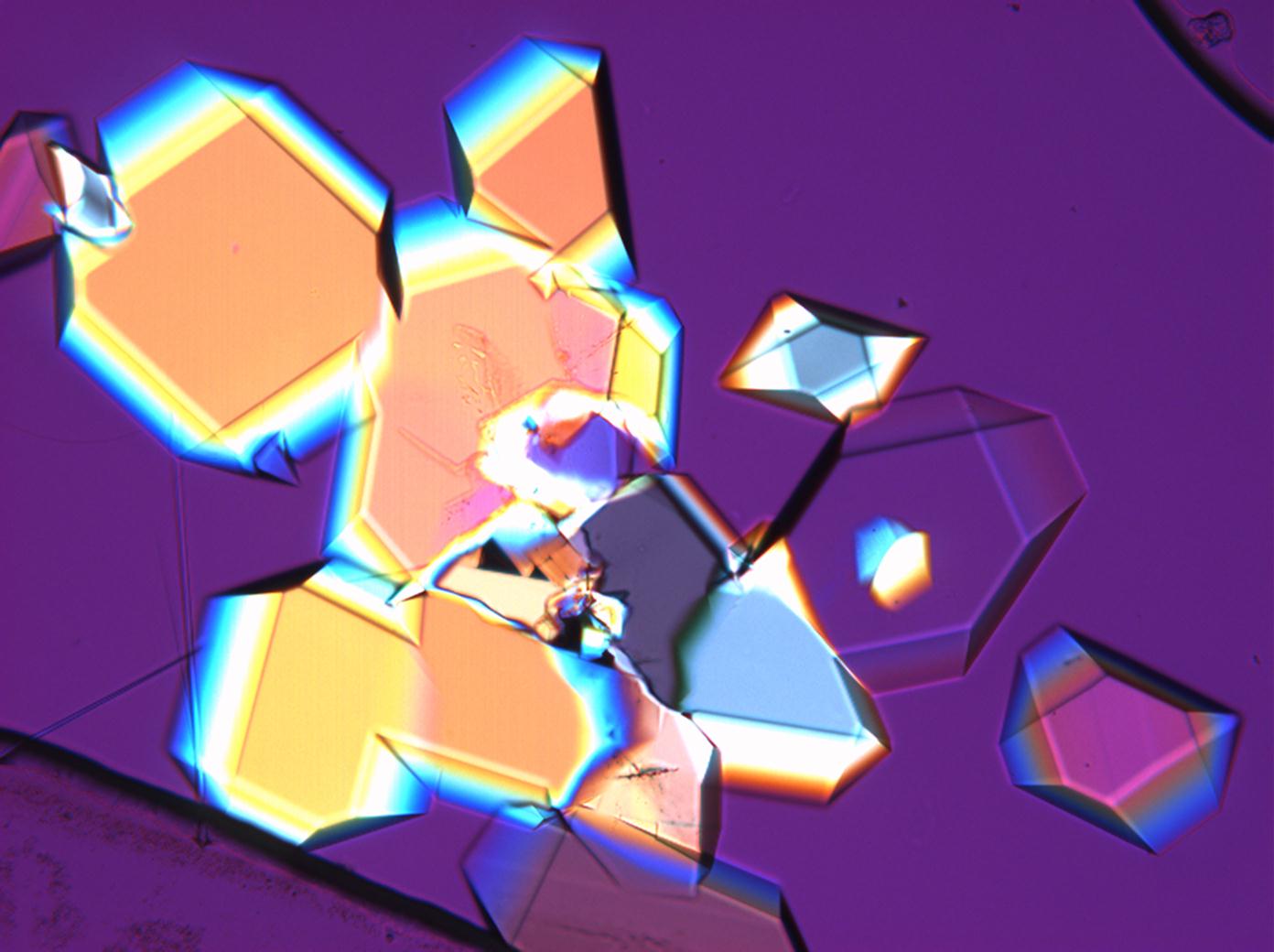
| |
| Mineralogical name | Mirabilite |
| Chemical name | Sodiumsulphate decahydrate |
| Trivial name | Glauber salt, Reussin, Sulphate of Soda |
| Chemical formula | Na2SO4•10H2O |
| Other forms | Sodiumsulphate heptahydrate Na2SO4•7H2O |
| Crystal system | monoclinic |
| Crystal structure | |
| Deliquescence humidity 20°C | 93,6% (20°C), 90% (23°C), 87% (25°C) |
| Solubility (g/l) at 20°C | 900 g/l |
| Density (g/cm³) | 1,464 g/cm³ |
| Molar volume | 219,8 cm3/mol |
| Molar weight | 322,21 g/mol |
| Transparency | transparent to opaque |
| Cleavage | perfect |
| Crystal habit | |
| Twinning | |
| Phase transition | |
| Chemical behavior | |
| Comments | soluble in water and glycerin, not soluble in pure alcohol easily loses some water, converts to thenardite at 32°C anormal blue or brown interference colours |
| Crystal Optics | |
| Refractive Indices | nx = 1,395 ny = 1,396-1,410 nz = 1,398-1,419 |
| Birefringence | Δ = 0,04-0,023 |
| Optical Orientation | negative |
| Pleochroism | |
| Dispersion | |
| Used Literature | |
| {{{Literature}}} | |
back to Sulphate
Weblinks[edit]
- ↑ http://webmineral.com/data/Mirabilite.shtml viewed on 29/07/2010
- ↑ http://www.mindat.org/min-2725.html viewed on 29/07/2010