Deterioration Patterns Wallpaintings: Difference between revisions
No edit summary |
No edit summary |
||
| Line 1: | Line 1: | ||
<!--Englisch Version by Hans-Jürgen Schwarz and Sandra Leithäuser--> | |||
<bibimport/> | <bibimport/> | ||
Author: [[user:NRiedl|Nicole Riedl]]<br><br> | Author: [[user:NRiedl|Nicole Riedl]]<br><br> | ||
back to [[Decay Pattern]] | back to [[Decay Pattern]] | ||
<br> | <br> | ||
| Line 11: | Line 10: | ||
== Examples of damage by salt progression == | == Examples of damage by salt progression == | ||
== | == Calcareous sinter crusts == | ||
[[file:Nehren Kalksinterkruste.jpg|thumb|300px|left| | [[file:Nehren Kalksinterkruste.jpg|thumb|300px|left| Calcareous sinter crust, Roman wall-painting, grave chamber, Nehren]] | ||
[[file:Kalksinterkruste NWW Nehren.jpg|thumb|300px|right| | [[file:Kalksinterkruste NWW Nehren.jpg|thumb|300px|right| Calcareous sinter crust, Roman wall-painting, grave chamber Nehren]] | ||
Repeated precipitation of calcium carbonate | Repeated precipitation of calcium carbonate on the surface of wall paintings forms a white, dense calcareous sinter crust. It is characterized by a high stability and strong bond with the painting. The vapor permeability of the wall painting is reduced and the layered crust deposits affect the aesthetic appearance considerably. | ||
<br clear=all> | <br clear=all> | ||
== Cauliflower crust == | == Cauliflower crust == | ||
[[file:Blumenkohlkruste Nehren NWW Detail.jpg|thumb|300px|left|Cauliflower crust, | [[file:Blumenkohlkruste Nehren NWW Detail.jpg|thumb|300px|left|Cauliflower crust, grave chamber Nehren]] | ||
[[file:Blumenkohlkruste Nehren NWW.jpg|thumb|300px|right| | [[file:Blumenkohlkruste Nehren NWW.jpg|thumb|300px|right|Cauliflower crust, grave chamber Nehren]] | ||
The formation of firmly adhering pustules and stable crust deposits, is a characteristic of the cauliflower crust. The composition may consist of calcium carbonate or calcium sulphate. Depending on the contaminants present, the cauliflower crusts appear whitish, yellowish, reddish or brownish. Biogenic precipitate can be stored within the crust deposits. | |||
<br clear=all> | <br clear=all> | ||
== Hygroscopic salts == | == Hygroscopic salts == | ||
[[file:Gipskruste Hygroskopisch.jpg|thumb|300px|left| | [[file:Gipskruste Hygroskopisch.jpg|thumb|300px|left|Gypsum crust, hygroscopic, grave chamber Nehren]] | ||
[[file:Salzkruste Hygroskopisch Nehren.jpg|thumb|300px|right| | [[file:Salzkruste Hygroskopisch Nehren.jpg|thumb|300px|right|Gypsum crust, hygroscopic, grave chamber Nehren]] | ||
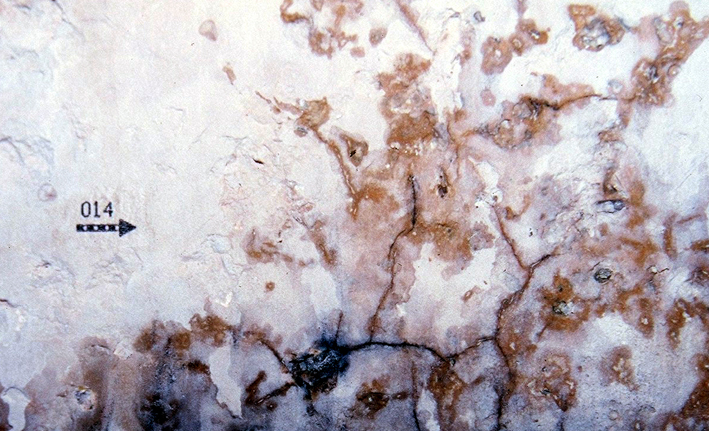
Darkened areas within the layers of crust deposits are visible alongside fissures in the paint layer and the topcoat of the plaster. Increased humidity, originating from the plaster or the ambient air, is bound by the salt crystals and appears macroscopically as a dark, blotched composition. | |||
<br clear=all> | <br clear=all> | ||
== Powdery salt deposits == | == Powdery salt deposits == | ||
[[file:PulvrigeSalzkristalle-01.jpg|thumb|300px|left| | [[file:PulvrigeSalzkristalle-01.jpg|thumb|300px|left| Powdery salt efflorescence, sodium sulfate, grave chamber Nehren]] | ||
[[file:PulvrigeSalzkristalle-Natriumsulfat.jpg|thumb|300px|right| | [[file:PulvrigeSalzkristalle-Natriumsulfat.jpg|thumb|300px|right| Powdery salt efflorescence, sodium sulfate, grave chamber Nehren]] | ||
White, loosely adhering salt efflorescence on the plaster and the paint surface. A characteristic of this efflorescence is the formation of small crystalline, soft, powdery salts, which can differ considerably in their chemical composition. The example shows sodium sulfate deposits on a repair plaster made of trass cement. | |||
<br clear=all> | <br clear=all> | ||
== Scaling salt activity == | == Scaling due to salt activity == | ||
[[file:Schalenbildung Nehren-01.jpg|thumb|300px|left| | [[file:Schalenbildung Nehren-01.jpg|thumb|300px|left|Scaling, grave chamber Nehren]] | ||
[[file:Schalenbildung Nehren-02.jpg|thumb|300px|right| | [[file:Schalenbildung Nehren-02.jpg|thumb|300px|right|Scaling, grave chamber Nehren]] | ||
The crystallizing salts burst the plaster structure. During the phase transition from liquid salt solution to the formation of salt crystals, an expansion takes place and pressure is applied to the adjacent plaster matrix. Hence, entire layers of plaster are lifted from the substructure and become crumbly and fragile. | |||
<br clear=all> | <br clear=all> | ||
[[Category:NRiedl]][[Category:Decay Pattern:Wall Paintings]] [[Category:R-HJuling]] [[Category:R-CBlaeuer]] [[Category:inProgress]] | [[Category:NRiedl]][[Category:Decay Pattern:Wall Paintings]] [[Category:R-HJuling]] [[Category:R-CBlaeuer]] [[Category:inProgress]] | ||
Revision as of 19:19, 7 January 2012
<bibimport/>
Author: Nicole Riedl
back to Decay Pattern
Examples of damage by salt progression[edit]
Calcareous sinter crusts[edit]
Repeated precipitation of calcium carbonate on the surface of wall paintings forms a white, dense calcareous sinter crust. It is characterized by a high stability and strong bond with the painting. The vapor permeability of the wall painting is reduced and the layered crust deposits affect the aesthetic appearance considerably.
Cauliflower crust[edit]
The formation of firmly adhering pustules and stable crust deposits, is a characteristic of the cauliflower crust. The composition may consist of calcium carbonate or calcium sulphate. Depending on the contaminants present, the cauliflower crusts appear whitish, yellowish, reddish or brownish. Biogenic precipitate can be stored within the crust deposits.
Hygroscopic salts[edit]
Darkened areas within the layers of crust deposits are visible alongside fissures in the paint layer and the topcoat of the plaster. Increased humidity, originating from the plaster or the ambient air, is bound by the salt crystals and appears macroscopically as a dark, blotched composition.
Powdery salt deposits[edit]
White, loosely adhering salt efflorescence on the plaster and the paint surface. A characteristic of this efflorescence is the formation of small crystalline, soft, powdery salts, which can differ considerably in their chemical composition. The example shows sodium sulfate deposits on a repair plaster made of trass cement.
Scaling due to salt activity[edit]
The crystallizing salts burst the plaster structure. During the phase transition from liquid salt solution to the formation of salt crystals, an expansion takes place and pressure is applied to the adjacent plaster matrix. Hence, entire layers of plaster are lifted from the substructure and become crumbly and fragile.