Nitronatrite: Difference between revisions
Jump to navigation
Jump to search
Weblinks
No edit summary |
No edit summary |
||
| Line 1: | Line 1: | ||
Authtor: [[user:Hschwarz|Hans-Jürgen schwarz]] | |||
<br> | |||
back to [[Nitrate]] | |||
{{Infobox_Salt | {{Infobox_Salt | ||
|Footnote =<ref>http://webmineral.com/data/Nitratine.shtml seen on 29.07.2010</ref><ref>http://www.mindat.org/min-2916.html seen on 29.07.2010</ref> | |Footnote =<ref>http://webmineral.com/data/Nitratine.shtml seen on 29.07.2010</ref><ref>http://www.mindat.org/min-2916.html seen on 29.07.2010</ref> | ||
| Line 26: | Line 31: | ||
|chemBehavior = | |chemBehavior = | ||
|Comments = | |Comments = | ||
|Literature = | |||
}} | }} | ||
| Line 42: | Line 45: | ||
<br clear=all> | <br clear=all> | ||
{|border="2" cellspacing="0" cellpadding="4" width="52%" align="left" class="wikitable" | {|border="2" cellspacing="0" cellpadding="4" width="52%" align="left" class="wikitable" | ||
|+''Table 1: Deliquescence himdities of sodium nitrate at different round temperatures <bib id="Steiger etal: 2014"/>'' | |+''Table 1: Deliquescence himdities of sodium nitrate at different round temperatures <bib id="Steiger.etal:2014"/>'' | ||
|- | |- | ||
|bgcolor = "#F0F0F0" align=center| 0°C | |bgcolor = "#F0F0F0" align=center| 0°C | ||
| Line 73: | Line 76: | ||
==Literature== | ==Literature== | ||
< | <biblist/> | ||
[[Category:Nitronatrite]][[Category:Nitrate]][[Category:Salt]][[Category:InProgress]] | [[Category:Nitronatrite]][[Category:Nitrate]][[Category:Salt]][[Category:InProgress]][[Category:List]] | ||
Revision as of 17:11, 15 February 2015
Authtor: Hans-Jürgen schwarz
back to Nitrate
| Nitronatrite[1][2] | |
| |
| Mineralogical name | Sodium Nitrate |
| Chemical name | Natriumnitrat |
| Trivial name | Nitratine, Nitratite, Soda Niter, Cubic Niter |
| Chemical formula | NaNO3 |
| Other forms | |
| Crystal system | trigonal |
| Crystal structure | |
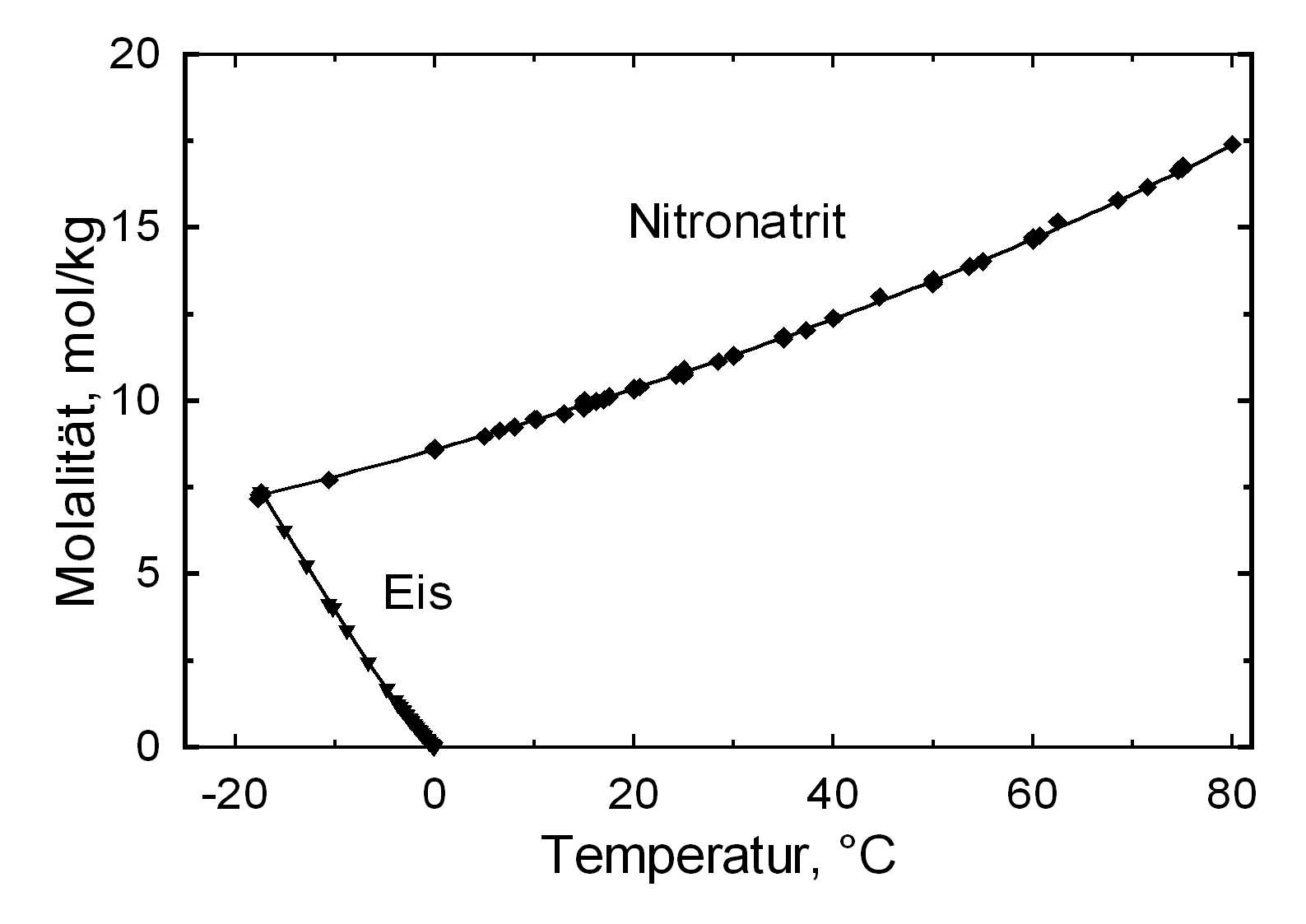
| Deliquescence humidity 20°C | 75.3% [Steiger etal: 2014]Title: Weathering and Deterioration Author: Steiger, Michael; Charola A. Elena; Sterflinger, Katja 
|
| Solubility (g/l) at 20°C | 880 g/l |
| Density (g/cm³) | 2.24-2.29 g/cm3 |
| Molar volume | 37.6 cm3/mol |
| Molar weight | 85 g/mol |
| Transparency | transparent |
| Cleavage | perfect |
| Crystal habit | |
| Twinning | |
| Phase transition | |
| Chemical behavior | |
| Comments | |
| Crystal Optics | |
| Refractive Indices | no = 1.587 ne = 1.336 |
| Birefringence | Δ = 0.251 |
| Optical Orientation | |
| Pleochroism | |
| Dispersion | |
| Used Literature | |
Solubility properties[edit]
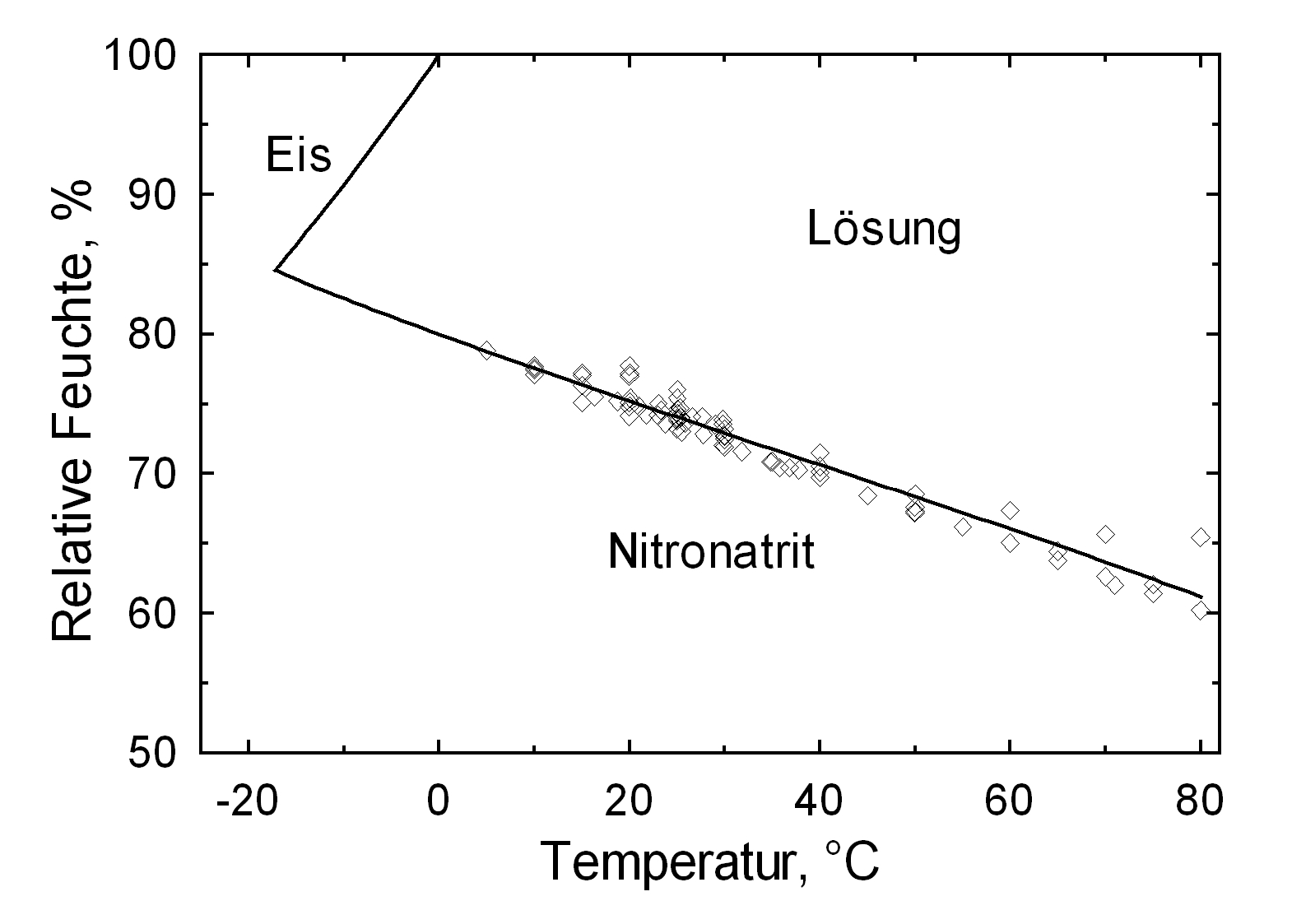
Hygroscopicity[edit]
| 0°C | 10°C | 20°C | 30°C | 40°C | 50°C |
| 80.1%r.h. | 77.7%r.h. | 75.3%r.h. | 72.8%r.h. | 70.4%r.h. | 68.0%r.h. |
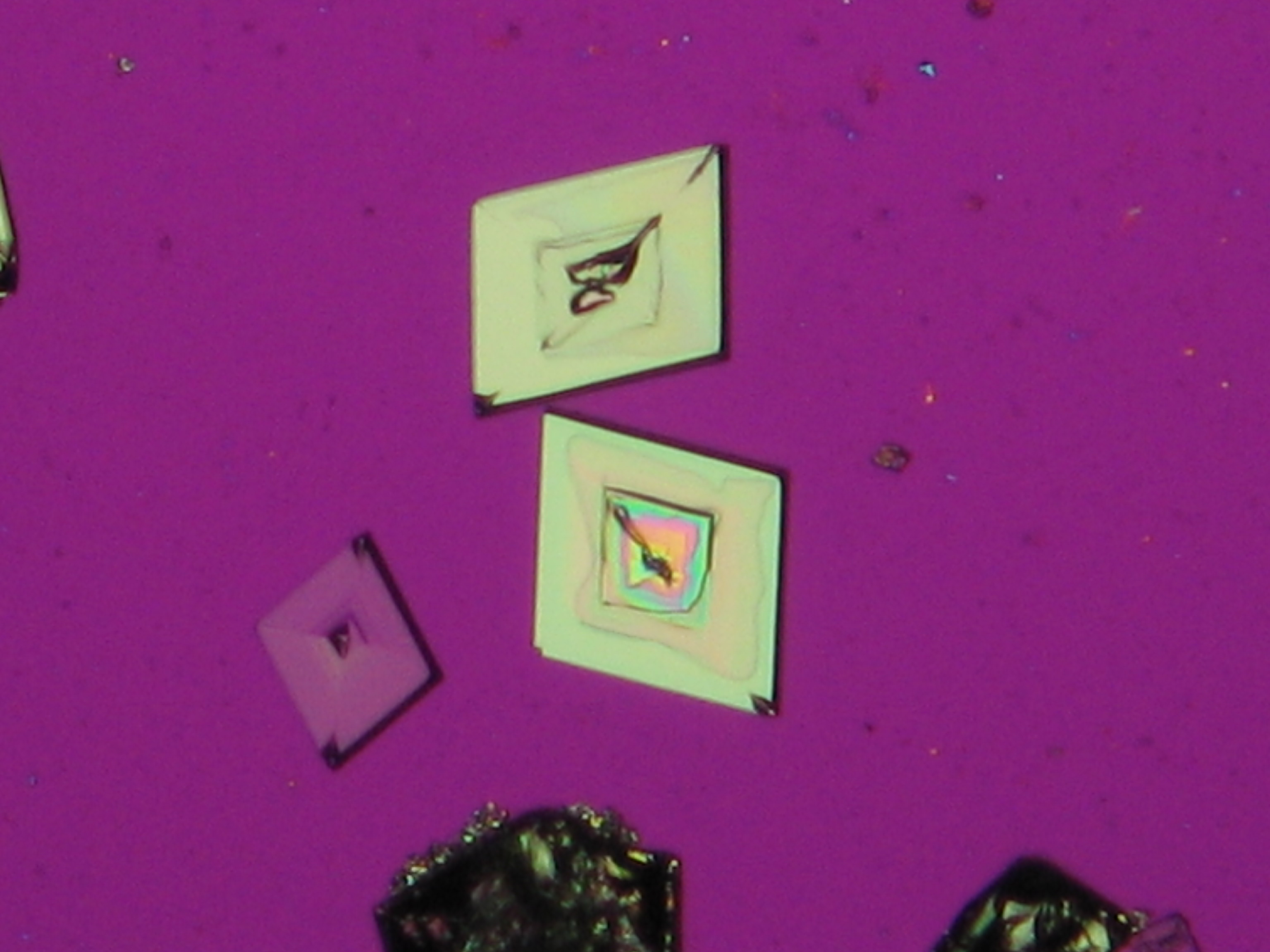
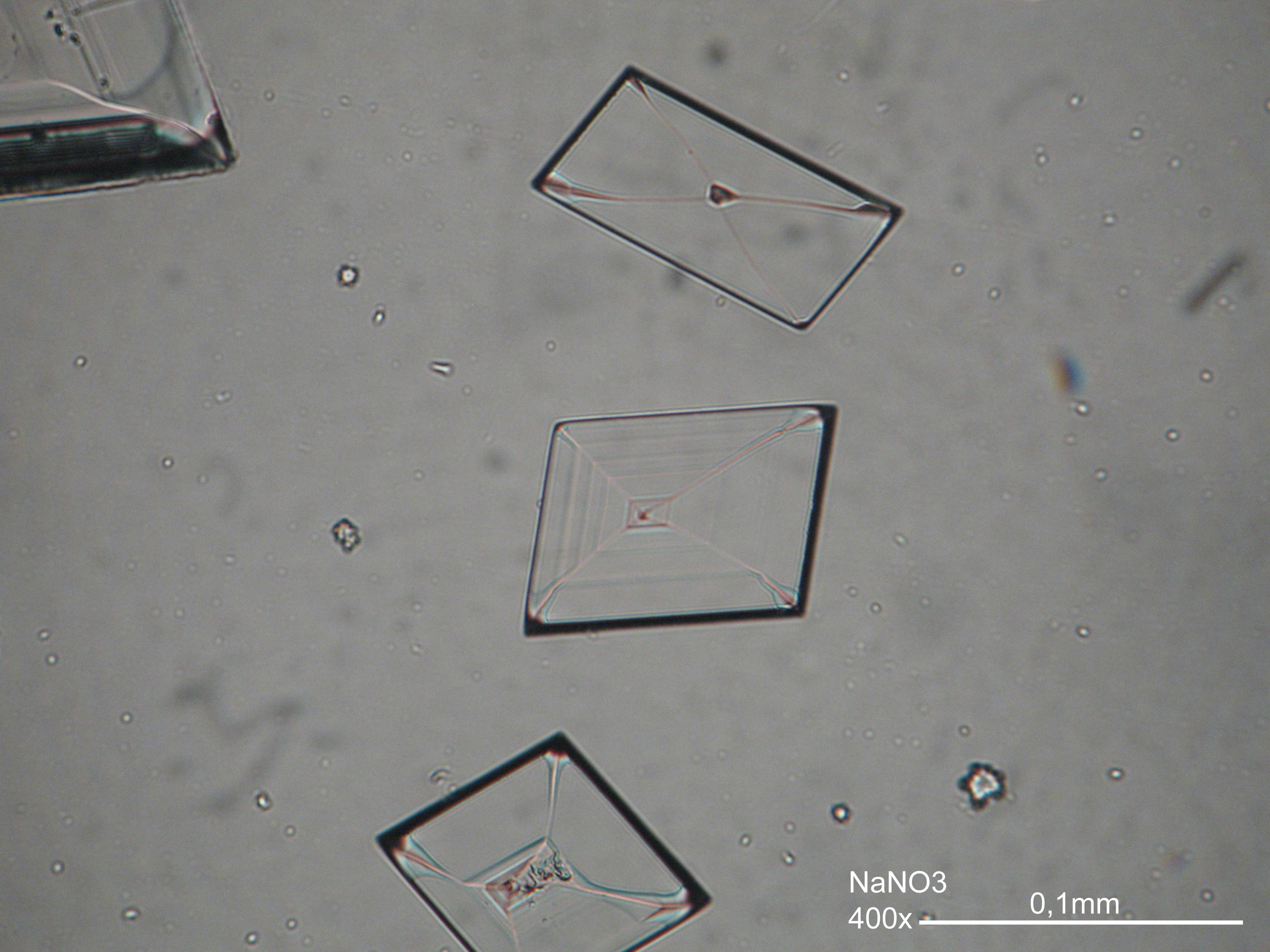
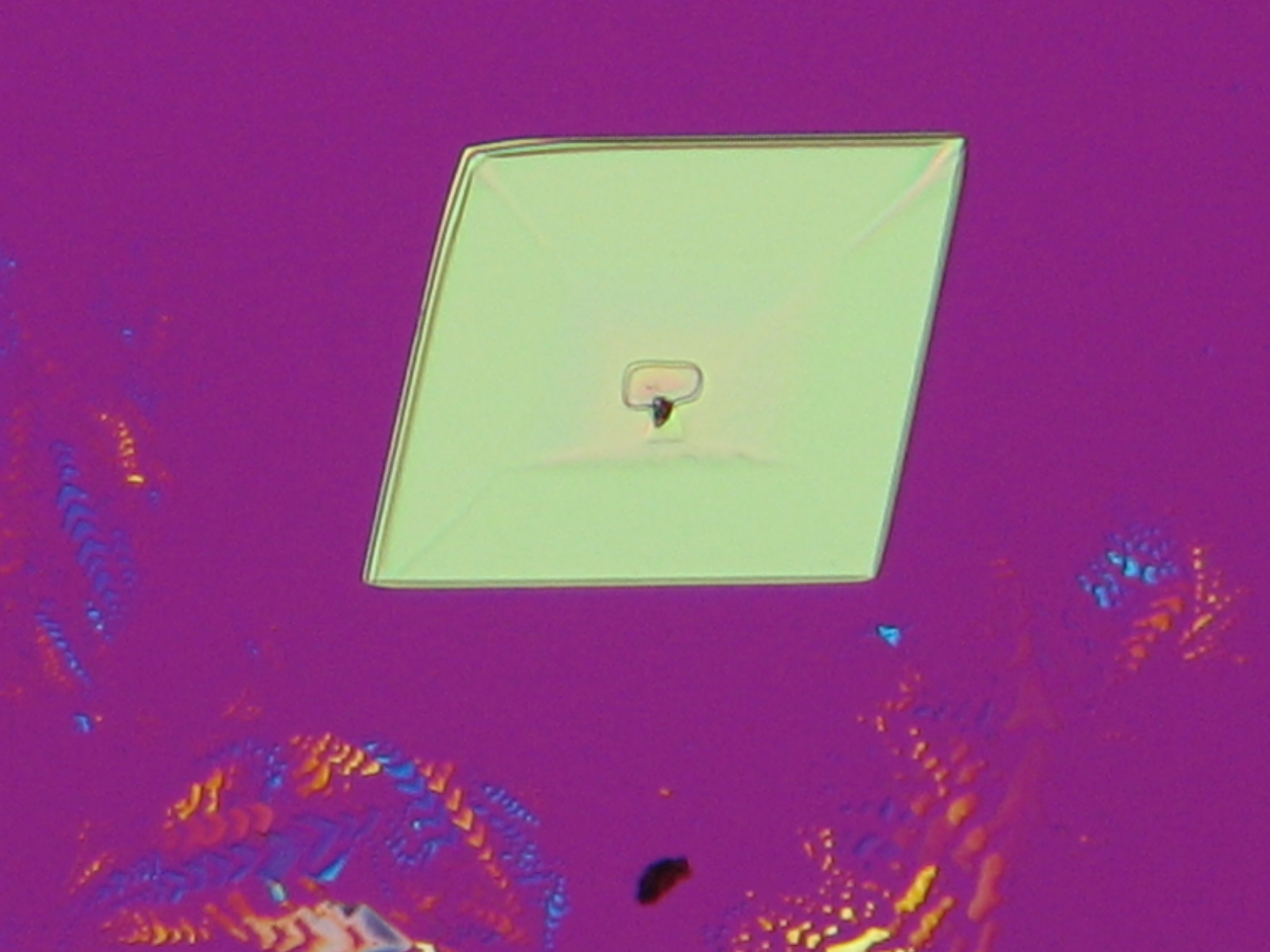
Under the polarising microscope[edit]
- crystallized on a glass slide
Weblinks
[edit]
- ↑ http://webmineral.com/data/Nitratine.shtml seen on 29.07.2010
- ↑ http://www.mindat.org/min-2916.html seen on 29.07.2010
Literature[edit]
| [Robie.etal:1978] | Robie R.A., Hemingway B.S.; Fisher J.A. (1978): Thermodynamic properties of minerals and related substances at 298.15 K and 1 bar pressure and higher temperatures. In: U.S. Geol. Surv. Bull, 1452 () |  |
| [Steiger.etal:2014] | Steiger, Michael; Charola A. Elena; Sterflinger, Katja (2014): Weathering and Deterioration. In: Siegesmund S.; Snethlage R. (eds.): Stone in Architecture, Springer Verlag Berlin Heidelberg, 223-316, 10.1007/978-3-642-45155-3_4. |  |