Calicum nitrate: Difference between revisions
No edit summary |
|||
| Line 3: | Line 3: | ||
back to [[Nitrate]] | back to [[Nitrate]] | ||
{{Infobox_Salt | {{Infobox_Salt | ||
| Line 51: | Line 50: | ||
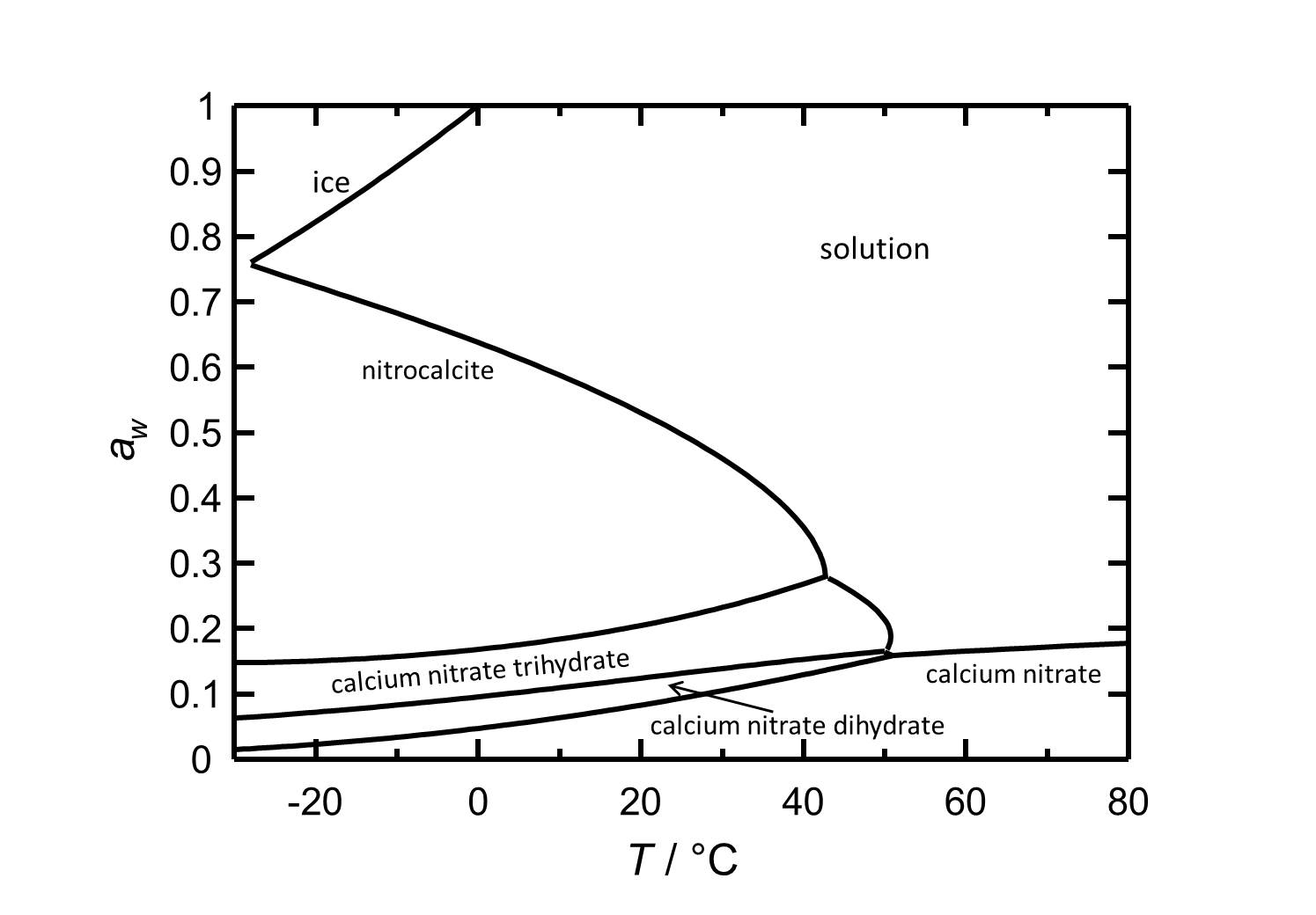
[[Image:Del Ca(NO3)2 e.jpg|thumb|left|800px|Figure 2: Deliquescence behaviour of calcium nitrate in the temperature range from -30 to 80 °C. The water acitivity ''a<sub>w</sub>'' is plotted against the temperature.]] | [[Image:Del Ca(NO3)2 e.jpg|thumb|left|800px|Figure 2: Deliquescence behaviour of calcium nitrate in the temperature range from -30 to 80 °C. The water acitivity ''a<sub>w</sub>'' is plotted against the temperature.]] | ||
<br clear=all> | <br clear=all> | ||
Calcium | Calcium nitrate and its phases are hygroscopic salts. At a temperature of 25 °C the [[Nitrocalcite]] has a deliquescence humidity of about 50 %. The phase transitions to the other two hydrated forms and to the anhydrous calcium nitrate occur at relative humidities of 22 %, 13 % and 9 %, respectively. | ||
==References== | ==References== | ||
Revision as of 16:21, 25 February 2015
Author: Amelie Stahlbuhk
back to Nitrate
| Calicum nitrate | |
| Mineralogical name | Calcium nitrate |
| Chemical name | Calcium nitrate |
| Trivial name | |
| Chemical formula | Ca(NO3)2 |
| Other forms | Ca(NO3)2•2H2O (Calcium nitrate dihydrate) Ca(NO3)2•3H2O (Calcium nitrate trihydrate) Ca(NO3)2•4H2O (Nitrocalcite) |
| Crystal system | |
| Crystal structure | |
| Deliquescence humidity 20°C | |
| Solubility (g/l) at 20°C | |
| Density (g/cm³) | 2.483 g/cm3 |
| Molar volume | 66.09 cm3/mol |
| Molar weight | 164.09 g/mol |
| Transparency | |
| Cleavage | |
| Crystal habit | |
| Twinning | |
| Phase transition | |
| Chemical behavior | |
| Comments | |
| Crystal Optics | |
| Refractive Indices | |
| Birefringence | |
| Optical Orientation | |
| Pleochroism | |
| Dispersion | |
| Used Literature | |
| [Robie.etal:1978]Title: Thermodynamic properties of minerals and related substances at 298.15 K and 1 bar pressure and higher temperatures Author: Robie R.A., Hemingway B.S.; Fisher J.A. 
| |
Abstract[edit]
In this article the salt calcium nitrate will be presented. The behavior regarding solubility an hygroscopicity will be shown for caclium nitrate and the different hydrate states.
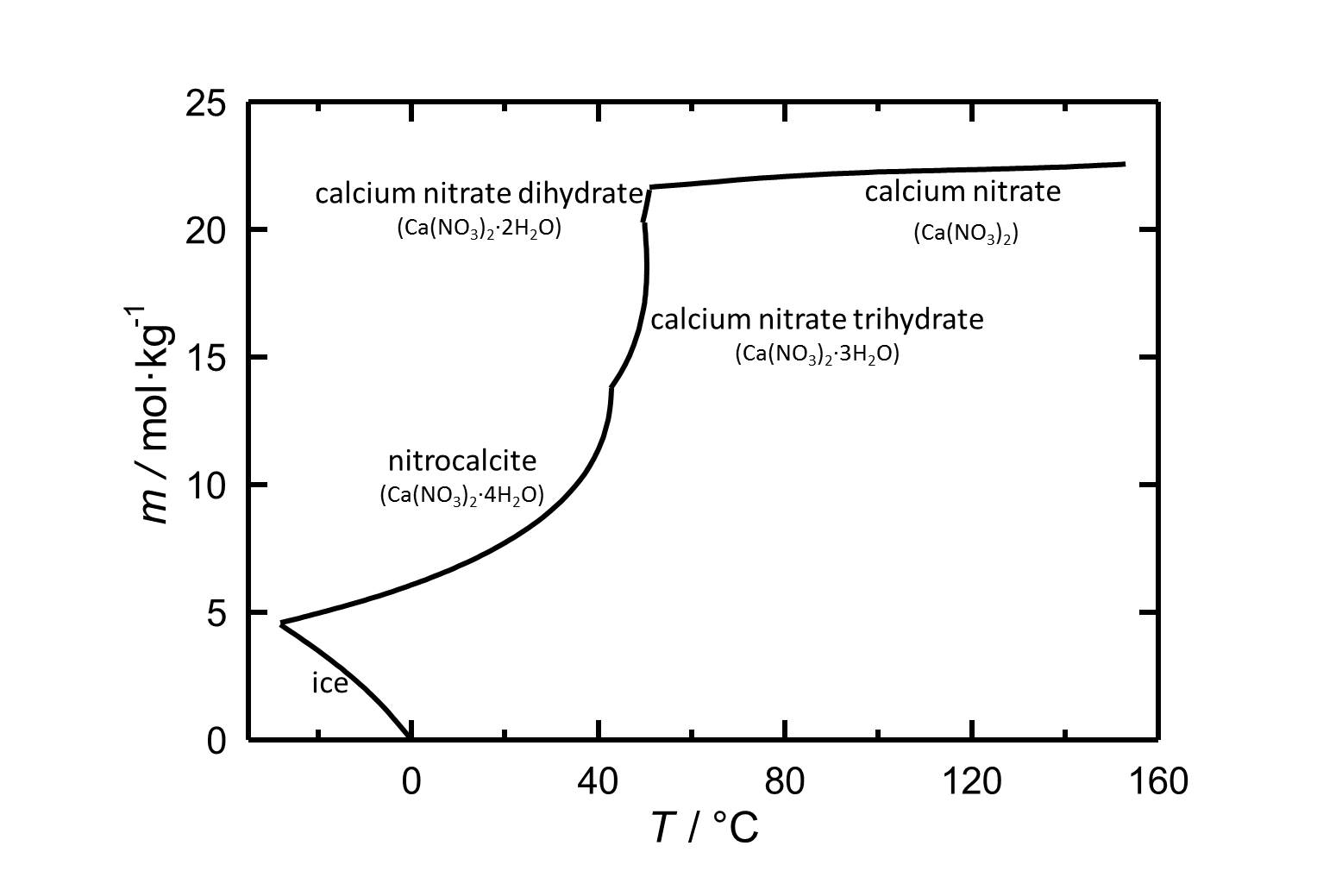
Solubility[edit]
Under standard conditions the tetrahydrate of calcium nitrate Nitrocalcite is the stable phase. With its relatively high solubility in water it is a highly soluble salt. The temperature dependence of the solubility is shown in the solubility diagram, where in parts the solubility increases extremely with increasing temperature. The dehydrations to the Trihydrate, Dihydrate and at least to the anhydrous calcium nitrate take place at 43 °C, 49.5 °C and 51 °C, respectively.
Hygroscopcity[edit]
Calcium nitrate and its phases are hygroscopic salts. At a temperature of 25 °C the Nitrocalcite has a deliquescence humidity of about 50 %. The phase transitions to the other two hydrated forms and to the anhydrous calcium nitrate occur at relative humidities of 22 %, 13 % and 9 %, respectively.
References[edit]
Literature[edit]
| [Robie.etal:1978] | Robie R.A., Hemingway B.S.; Fisher J.A. (1978): Thermodynamic properties of minerals and related substances at 298.15 K and 1 bar pressure and higher temperatures. In: U.S. Geol. Surv. Bull, 1452 () |  |