Nitrocalcite: Difference between revisions
Jump to navigation
Jump to search
Weblinks
No edit summary |
No edit summary |
||
| Line 14: | Line 14: | ||
|MolVolume = 129,8 cm<sup>3</sup>/mol | |MolVolume = 129,8 cm<sup>3</sup>/mol | ||
|Molweight = 236,16 g/mol | |Molweight = 236,16 g/mol | ||
|Transparency = | |Transparency = transparent | ||
|Cleavage = | |Cleavage = | ||
|Crystal_Habit = | |Crystal_Habit = | ||
Revision as of 12:11, 9 June 2011
| Nitrocalcite[1] | |
| |
| Mineralogical name | Nitrocalcite |
| Chemical name | Calcium Nitrate Tetrahydrate |
| Trivial name | Nitrate of lime |
| Chemical formula | Ca(NO3)2•4H2O |
| Other forms | |
| Crystal system | {{{Crystal_System}}} |
| Crystal structure | |
| Deliquescence humidity 20°C | 53,6 |
| Solubility (g/l) at 20°C | |
| Density (g/cm³) | 1,82 g/cm3 |
| Molar volume | 129,8 cm3/mol |
| Molar weight | 236,16 g/mol |
| Transparency | transparent |
| Cleavage | |
| Crystal habit | |
| Twinning | |
| Phase transition | |
| Chemical behavior | |
| Comments | |
| Crystal Optics | |
| Refractive Indices | nx = 1,465 ny = 1,498 nz = 1,504 |
| Birefringence | Δ = 0,039 |
| Optical Orientation | negative |
| Pleochroism | |
| Dispersion | |
| Used Literature | |
| {{{Literature}}} | |
back to Nitrate
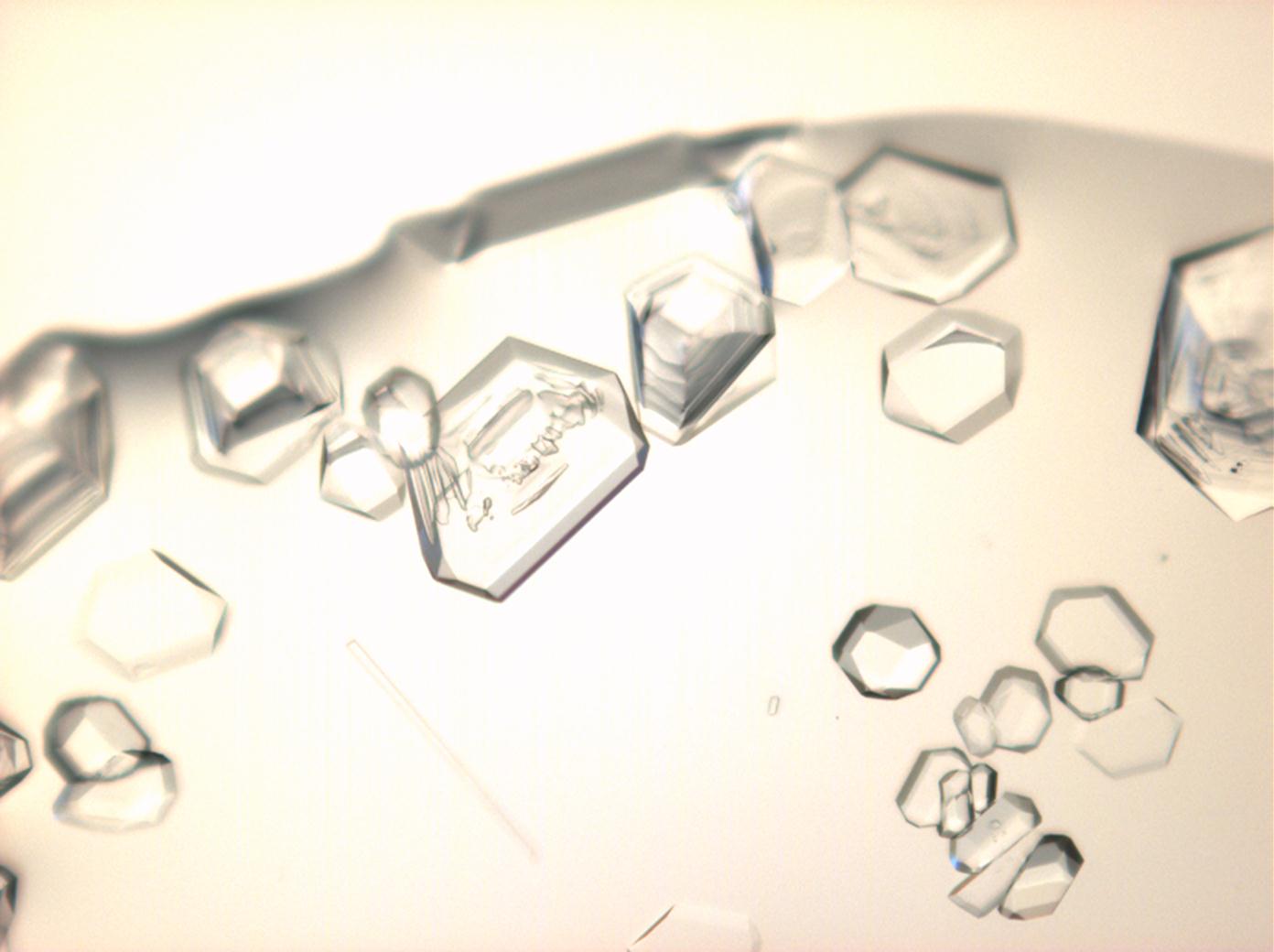
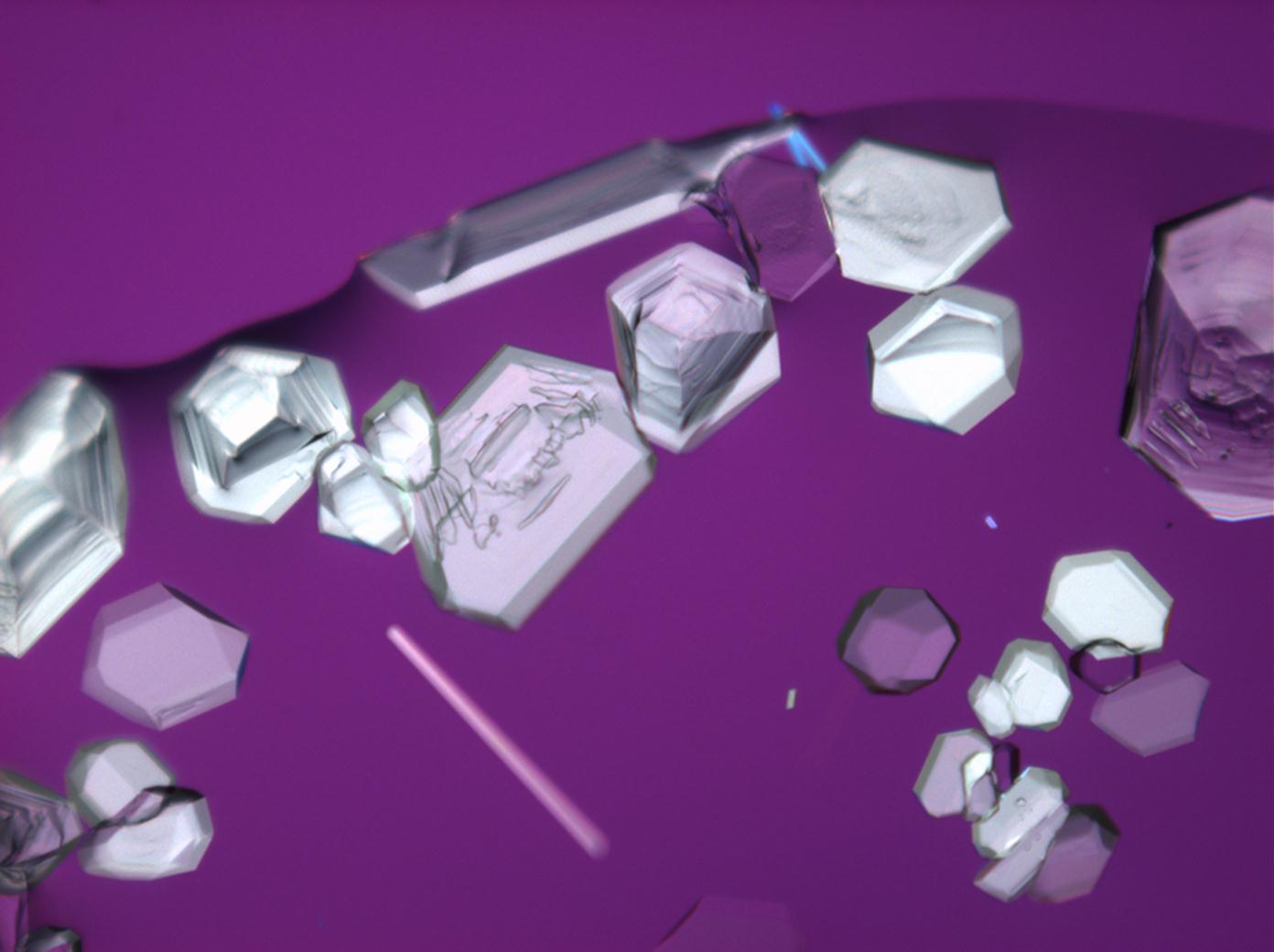
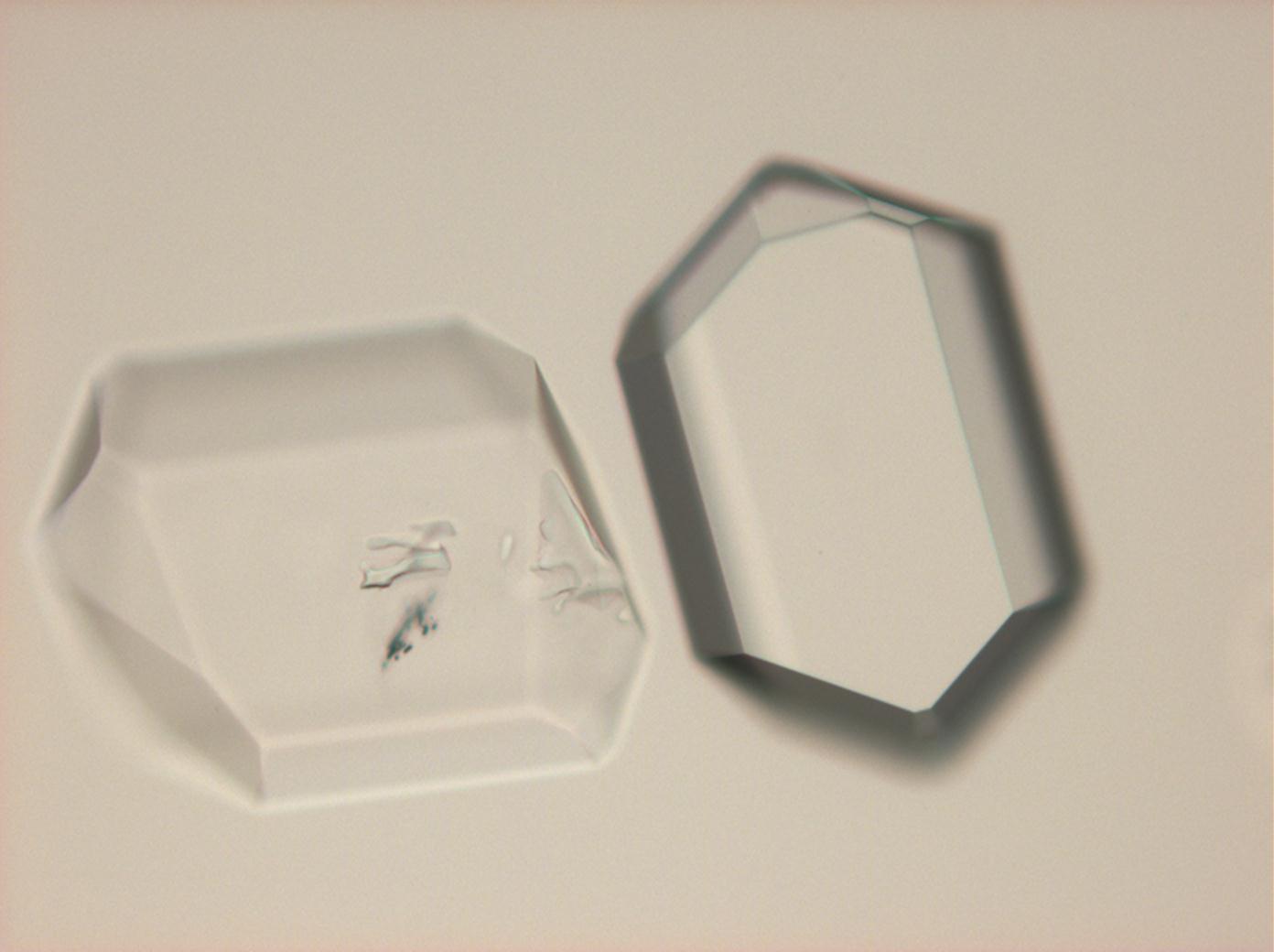
Under the polarising microscope[edit]
- Crysallised from a saturated solution with ethanol addition
- Crystallised in a micro climate chamber
Weblinks
[edit]
- ↑ http://www.mindat.org/min-2919.html seen on 29.07.2010