Aphthitalite: Difference between revisions
Jump to navigation
Jump to search
m (Protected "Aphthitalite" ([edit=sysop] (indefinite) [move=sysop] (indefinite))) |
No edit summary |
||
| Line 11: | Line 11: | ||
|Deliqueszenzhumidity = | |Deliqueszenzhumidity = | ||
|Solubility = | |Solubility = | ||
|Density = 2 | |Density = 2.7 g/cm<sup>3</sup> | ||
|MolVolume = 246 | |MolVolume = 246.2 cm<sup>3</sup>/mol | ||
|Molweight = 332 g/mol | |Molweight = 332 g/mol | ||
|Transparency = transparent to opaque | |Transparency = transparent to opaque | ||
| Line 18: | Line 18: | ||
|Crystal_Habit = | |Crystal_Habit = | ||
|Twinning = | |Twinning = | ||
|Refractive_Indices = n<sub>o</sub> = 1 | |Refractive_Indices = n<sub>o</sub> = 1.487-1.491<br> n<sub>e</sub> = 1.492-1.499 | ||
|Birefringence = Δ = 0 | |Birefringence = Δ = 0.005-0.008 | ||
|optical_Orientation = positive | |optical_Orientation = positive | ||
|Pleochroism = | |Pleochroism = | ||
Revision as of 14:22, 20 December 2011
| Aphthitalite[1][2] | |
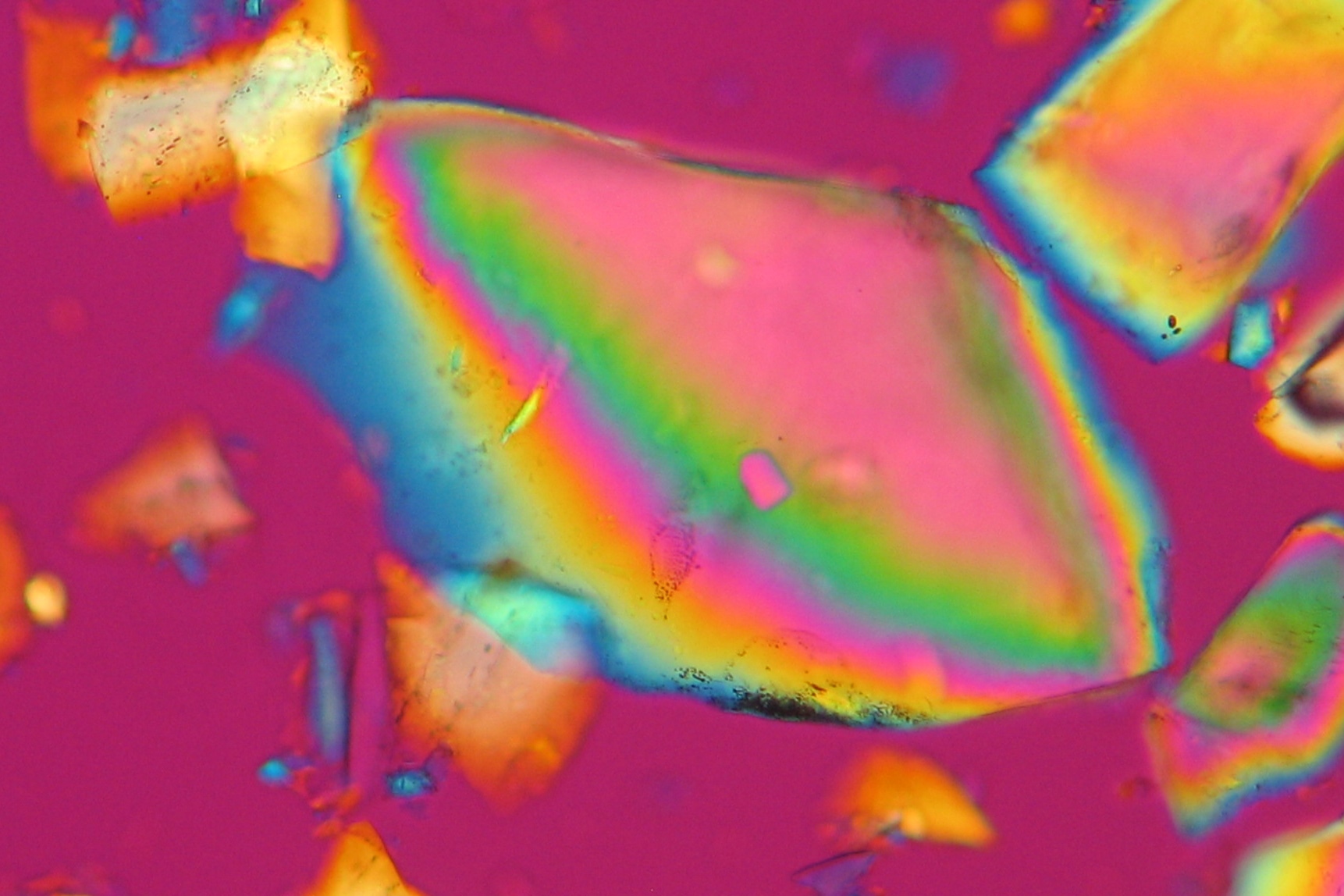
| |
| Mineralogical name | Aphthitalite |
| Chemical name | Potassium sodium sulphate |
| Trivial name | Glaserite, Vesuvian Salt |
| Chemical formula | K3Na(SO4)2 Na2SO4•3K2SO4 |
| Other forms | |
| Crystal system | trigonal |
| Crystal structure | |
| Deliquescence humidity 20°C | |
| Solubility (g/l) at 20°C | |
| Density (g/cm³) | 2.7 g/cm3 |
| Molar volume | 246.2 cm3/mol |
| Molar weight | 332 g/mol |
| Transparency | transparent to opaque |
| Cleavage | imperfect to fair |
| Crystal habit | |
| Twinning | |
| Phase transition | |
| Chemical behavior | |
| Comments | |
| Crystal Optics | |
| Refractive Indices | no = 1.487-1.491 ne = 1.492-1.499 |
| Birefringence | Δ = 0.005-0.008 |
| Optical Orientation | positive |
| Pleochroism | |
| Dispersion | |
| Used Literature | |
| {{{Literature}}} | |
back to Sulphate
Weblinks[edit]
- ↑ http://webmineral.com/data/Aphthitalite.shtml viewed on 29/07/2010
- ↑ http://www.mindat.org/min-280.html viewed on 29/07/2010