Talk:Thenardite: Difference between revisions
SLeithaeuser (talk | contribs) |
SLeithaeuser (talk | contribs) |
||
| Line 45: | Line 45: | ||
== Information on the origin and formation of thenardite / mirabilite in monuments == | == Information on the origin and formation of thenardite / mirabilite in monuments == | ||
With the entry of materials that contain soluble sodium compounds, the mineral system of a monument may create sodium sulfate as salt efflorescence, when reacting with various sources of sulfate for example air contaminated with sulphurous gases. In Germany cement exhibits a high content of sodium ions, because the German standardization institute (DIN) allows for a content of up to 0.5 % soluble alkalis. This means that 100 kg of Portland cement containing only 0.1% soluble Na<sub>2</sub>O can form 520g of [[Mirabilite]] when in contact with air containing sulfuric acid [calculation by Arnold/Zehnder 1991]. Sodium ions can also enter into monuments from a plethora of cleaning materials and especially older restoration products (such as water glass). Ground water and surface water are also a possible source of Na<sup>+</sup>-ions. Road salt consists to a large extent of slightly soluble [[Halite|sodium chloride]]. Finally, in | With the entry of materials that contain soluble sodium compounds, the mineral system of a monument may create sodium sulfate as salt efflorescence, when reacting with various sources of sulfate for example air contaminated with sulphurous gases. In Germany cement exhibits a high content of sodium ions, because the German standardization institute (DIN) allows for a content of up to 0.5 % soluble alkalis. This means that 100 kg of Portland cement containing only 0.1% soluble Na<sub>2</sub>O can form 520g of [[Mirabilite]] when in contact with air containing sulfuric acid [calculation by Arnold/Zehnder 1991]. Sodium ions can also enter into monuments from a plethora of cleaning materials and especially older restoration products (such as water glass). Ground water and surface water are also a possible source of Na<sup>+</sup>-ions. Road salt consists to a large extent of slightly soluble [[Halite|sodium chloride]]. Finally, in coastal areas, sea water is a significant source of [[Halite|NaCl]]. | ||
== Solubility behavior == | == Solubility behavior == | ||
Revision as of 13:54, 6 May 2012
| Thenardite[1][2] | |
| |
| Mineralogical name | Thenardite |
| Chemical name | Sodium sulfate |
| Trivial name | Pyrotechnite |
| Chemical formula | Na2SO4 |
| Other forms | Mirabilite (Na2SO4•10H2O) Sodiumsulfate heptahydrate (Na2SO4•7H2O) |
| Crystal system | orthorhombic |
| Crystal structure | |
| Deliquescence humidity 20°C | 81.7% (25°C) |
| Solubility (g/l) at 20°C | 162 g/l |
| Density (g/cm³) | 2.689 g/cm³ |
| Molar volume | 53.11 cm3/mol |
| Molar weight | 142.04 g/mol |
| Transparency | transparent to translucent |
| Cleavage | perfect |
| Crystal habit | |
| Twinning | |
| Phase transition | |
| Chemical behavior | |
| Comments | soluble in water and glycerin, not soluble in pure alcohol |
| Crystal Optics | |
| Refractive Indices | nx = 1.468 ny = 1.473 nz = 1.483 |
| Birefringence | Δ = 0.015 |
| Optical Orientation | positive |
| Pleochroism | |
| Dispersion | |
| Used Literature | |
| {{{Literature}}} | |
Authors: Hans-Jürgen Schwarz , Michael Steiger, Tim Müller
English translation by Matthieu Angeli
back to Sulfate
Sodium sulfate and thenardite[edit]
Abstract[edit]
Sodium sulfate and its phases thenardite, mirabilite and heptahydrate are described. Heptahydrate´s role as a major source of damage is also explained .
Occurrence[edit]
Both thenardite and mirabilite occur as natural minerals. In nature sodium sulfate occurs in mineral waters in the form of double salts, as deposits of former salt lakes. Knowledge of the hydrated sodium sulfate dates back to the 16th Century. Its first description has been written by Glauber in 1658 in which he described it as "sal mirable". It is also quite common to read the name "Glauber's salt" for mirabilite in the literature.
Information on the origin and formation of thenardite / mirabilite in monuments[edit]
With the entry of materials that contain soluble sodium compounds, the mineral system of a monument may create sodium sulfate as salt efflorescence, when reacting with various sources of sulfate for example air contaminated with sulphurous gases. In Germany cement exhibits a high content of sodium ions, because the German standardization institute (DIN) allows for a content of up to 0.5 % soluble alkalis. This means that 100 kg of Portland cement containing only 0.1% soluble Na2O can form 520g of Mirabilite when in contact with air containing sulfuric acid [calculation by Arnold/Zehnder 1991]. Sodium ions can also enter into monuments from a plethora of cleaning materials and especially older restoration products (such as water glass). Ground water and surface water are also a possible source of Na+-ions. Road salt consists to a large extent of slightly soluble sodium chloride. Finally, in coastal areas, sea water is a significant source of NaCl.
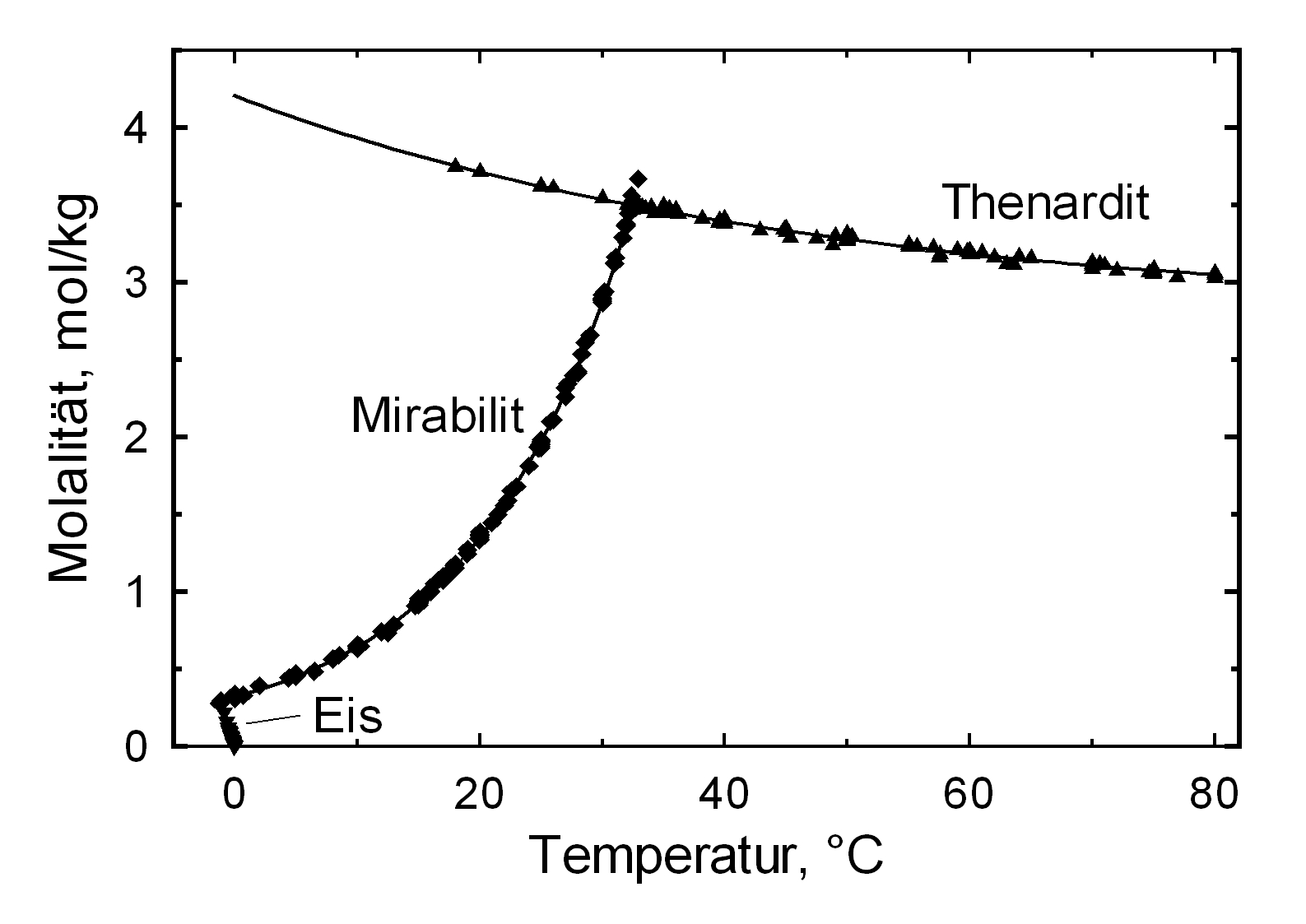
Solubility behavior[edit]
The structures of both thenardite and mirabilite belong to the group of easily soluble salts and therefore they are easily mobilized (see table hygroscopicity of the salts and ERH). The solubility of sodium sulfate is highly dependent on temperature. For this reason, a rapid drop of temperature is highly likely to yield very high supersaturation and salt crystallization.
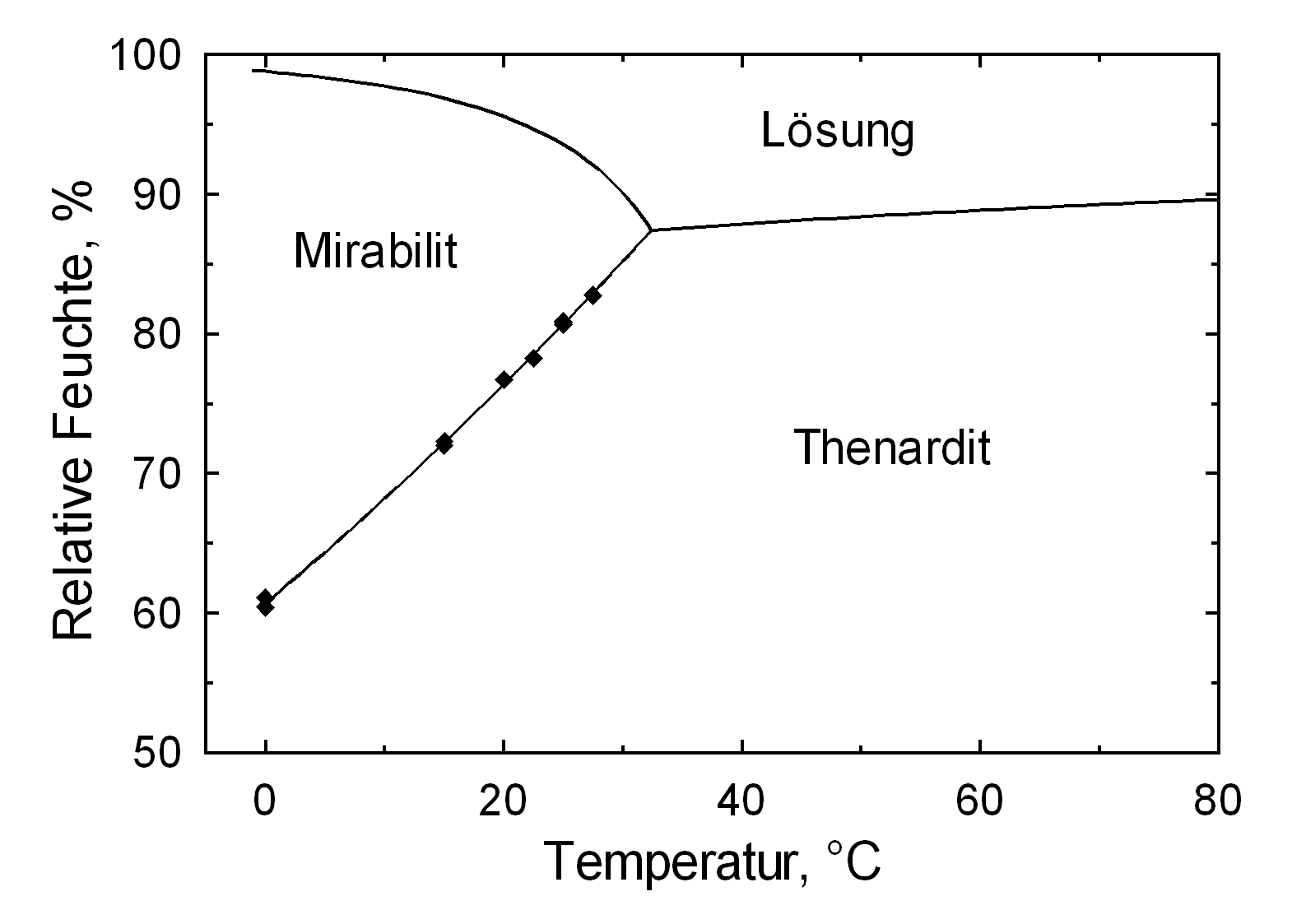
Hygroscopicity[edit]
The temperature effect on the deliquescence points of thenardite and mirabilite is shown below. The striking features here are the opposite curve transitions. In the presence of other ions (in salt mixtures), the parameters of the equilibrium moisture content, as well as the necessary temperature and humidity conditions for recrystallization, change significantly. The following table shows experimental data of equilibrium moisture for different salt mixtures at different temperatures.It turns out that all the values of equilibrium moisture content are lower than those of the pure salt mirabilite (see table equilibrium moisture content as a function of temperature).
| MgSO4 | Ca(NO3)2 | KNO3 | |
| Na2SO4 • 10H2O | 87(21°C) | 74 (21°C) | 81(21°C) |
Water vapor sorption:
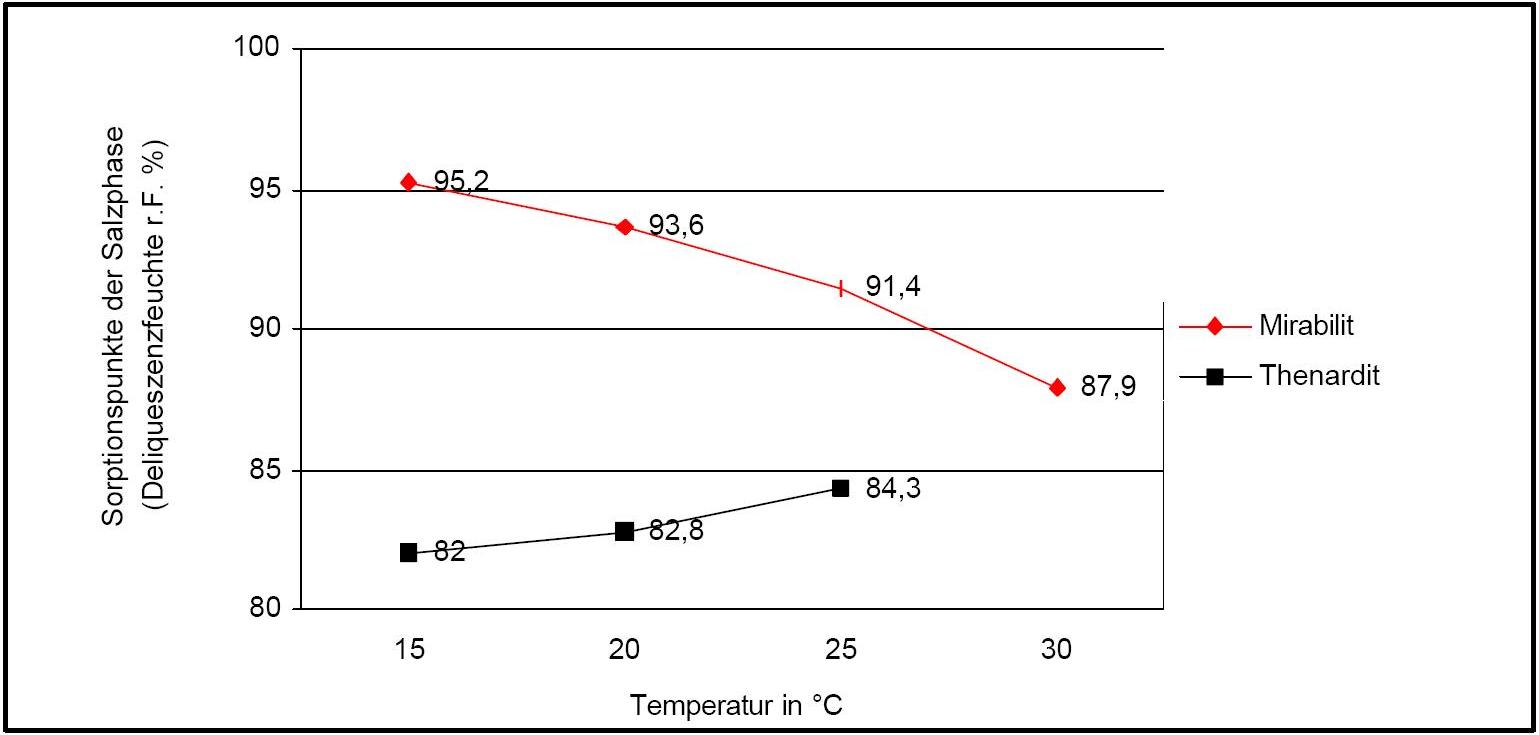
Author: Arnold, Andreas; Zehnder, Konrad

The table below shows additional information for estimating the hygroscopicity of sodium sulfate for the sorption behavior of pure salt and the mixture with halite at different relative humidity levels:
| Air humidity | 87% r.F. | 81% r.F. | 79% r.F. |
| Na2SO4 | 79 | 0 | 0 |
| Na2SO4+NaCl (1:1 molar mixture) | 157 | 32 | 15 |
Crystallization pressure[edit]
When crystallizing from an aqueous solution, the crystallization pressure of thenardite lies in the 29.2-34.5 N/mm2 range. These values are higher that those calculated for other building-damaging salts [Winkler:1975]Title: Stone: Properties, Durability in Man´s Environment
Author: Winkler, Erhard M. .
.
Hydration behavior[edit]
The Na2SO4 – H2O system:
The only stable forms of sodium sulfate are the decahydrate (mirabilite) and the anhydrite (thenardite). The generation of mirabilite by recrystallization of the salt from an aqueous supersaturated solution occurs at 32.4°C. In particular, the transition from thenardite to mirabilite and the incorporation of 10 water molecules in the crystal lattice causes a volume expansion of 320%. This transition happens at a relatively low temperature (32-35°C), the damage caused by this salt is highly dependent on the temperature and thus on the environment. This temperature range is given as a guide, because this transition could happen for example at 25°C at 80% relative humidity, or even at 0°C at 60.7% relative humidity [information from Gmelin]. Due to this strong dependence on the environmental parameters, an estimate of the damage caused on buildings by crystallization and hydration of sodium sulfate are very difficult to obtain.
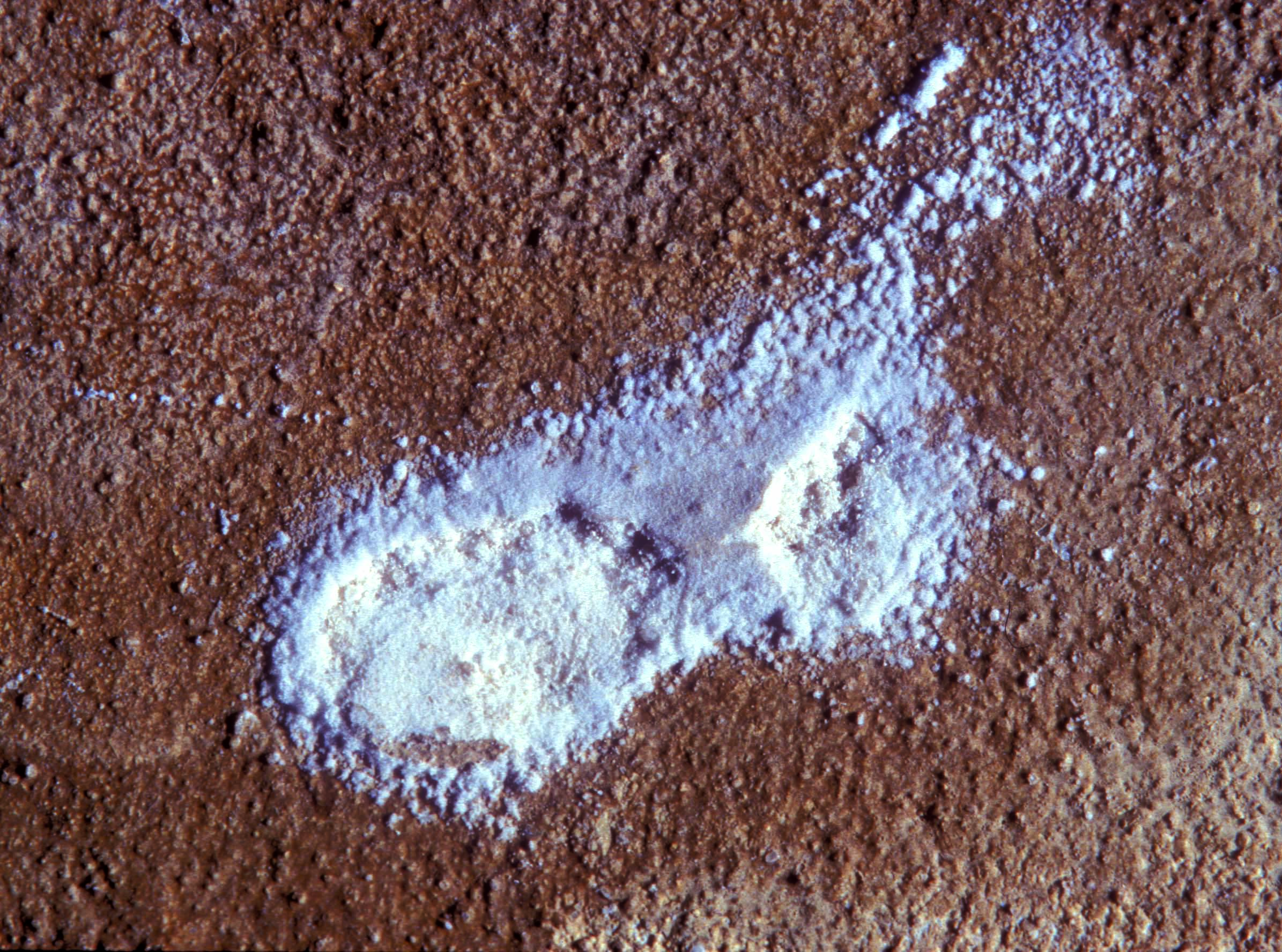
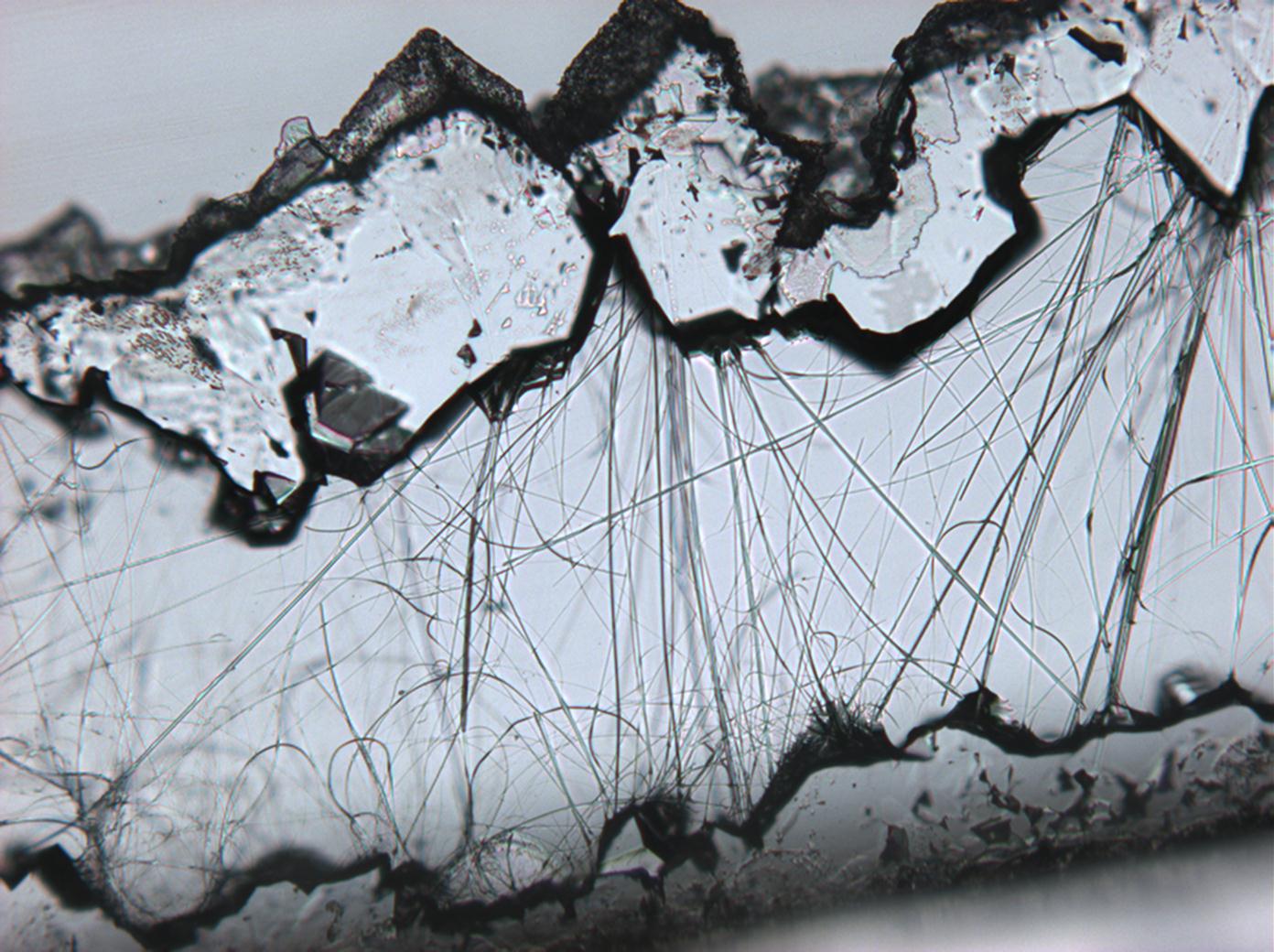
Pictures of salt and salt damage[edit]
In the field[edit]
- Thenardite efflorescences
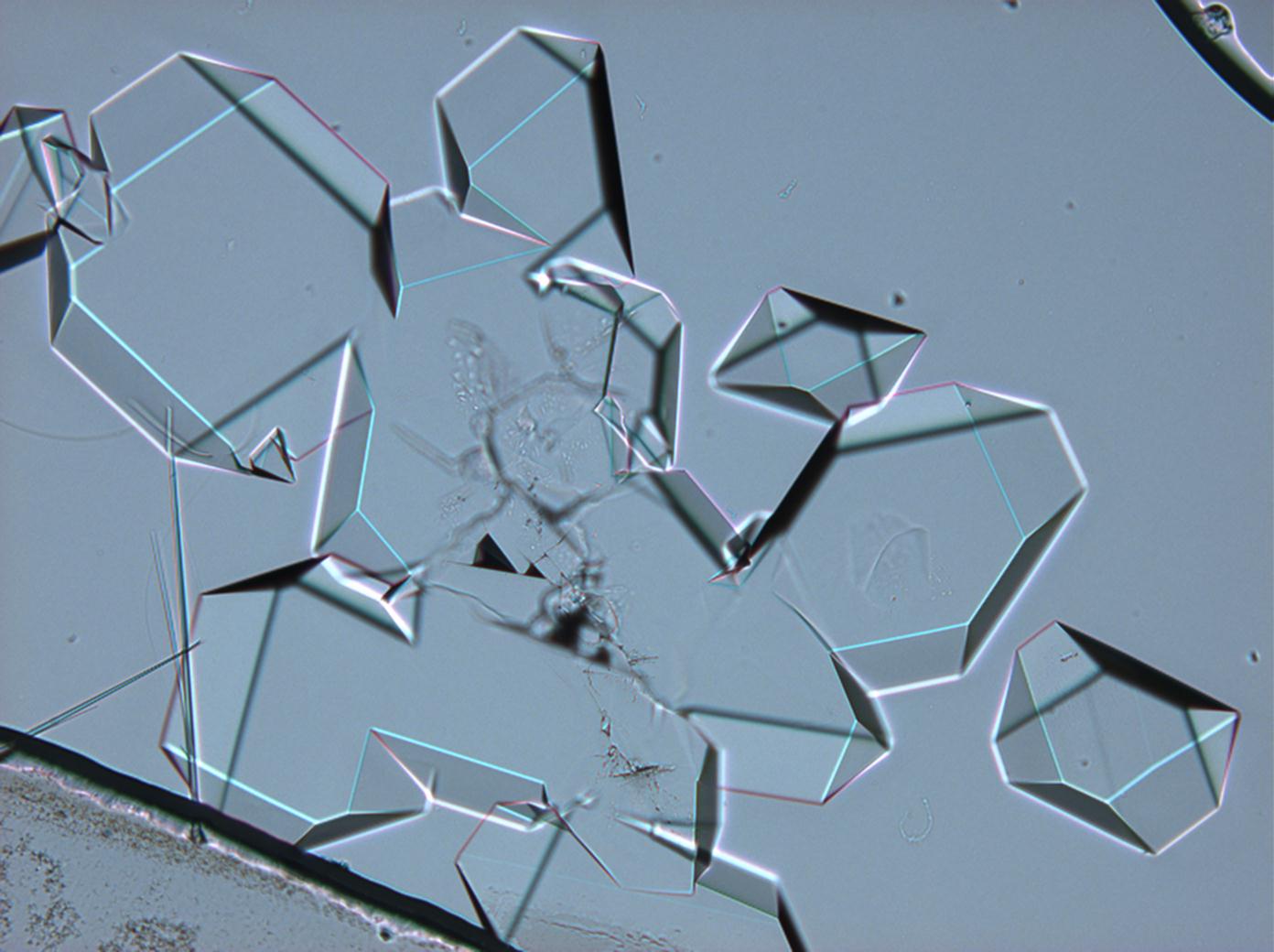
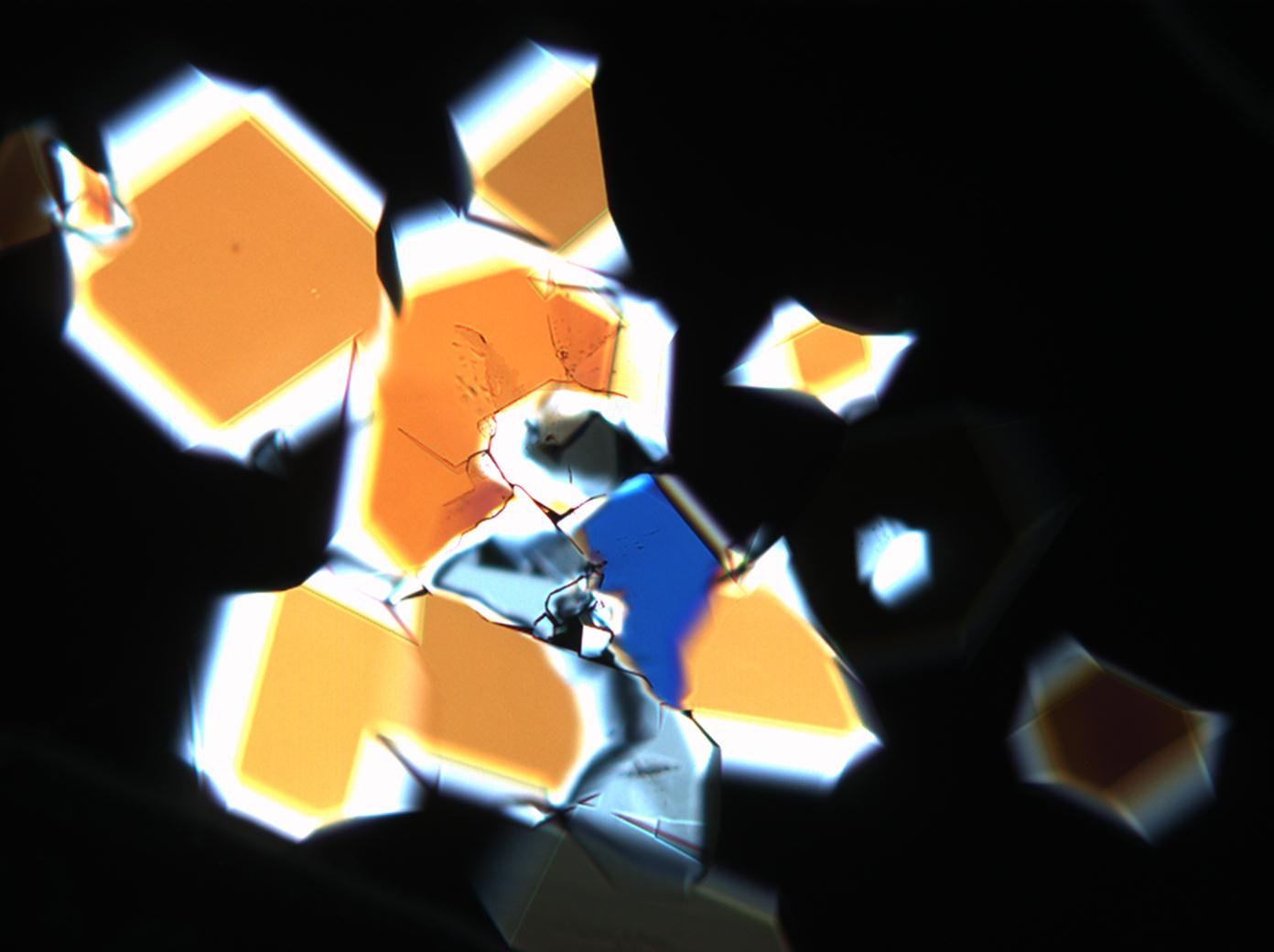
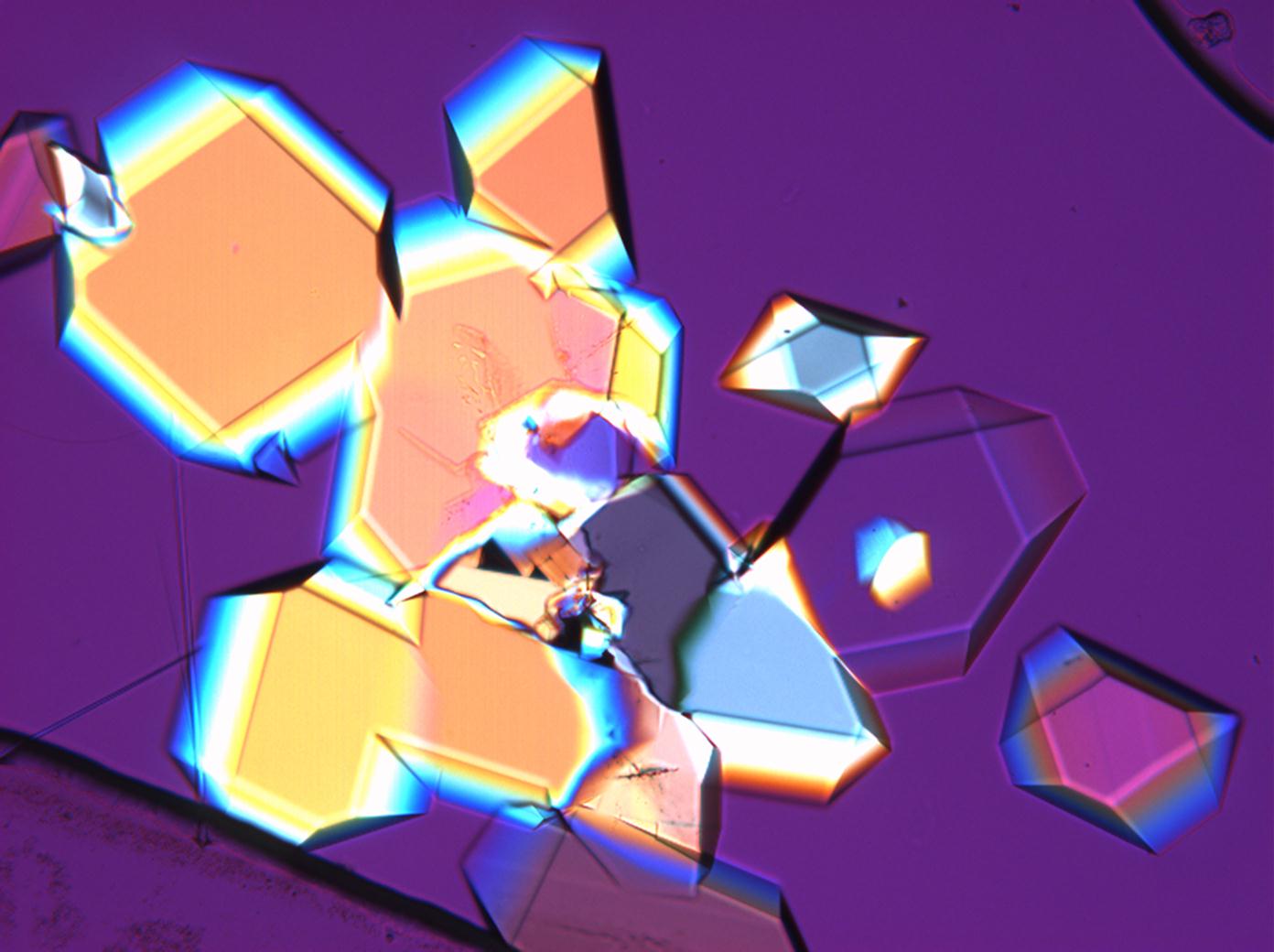
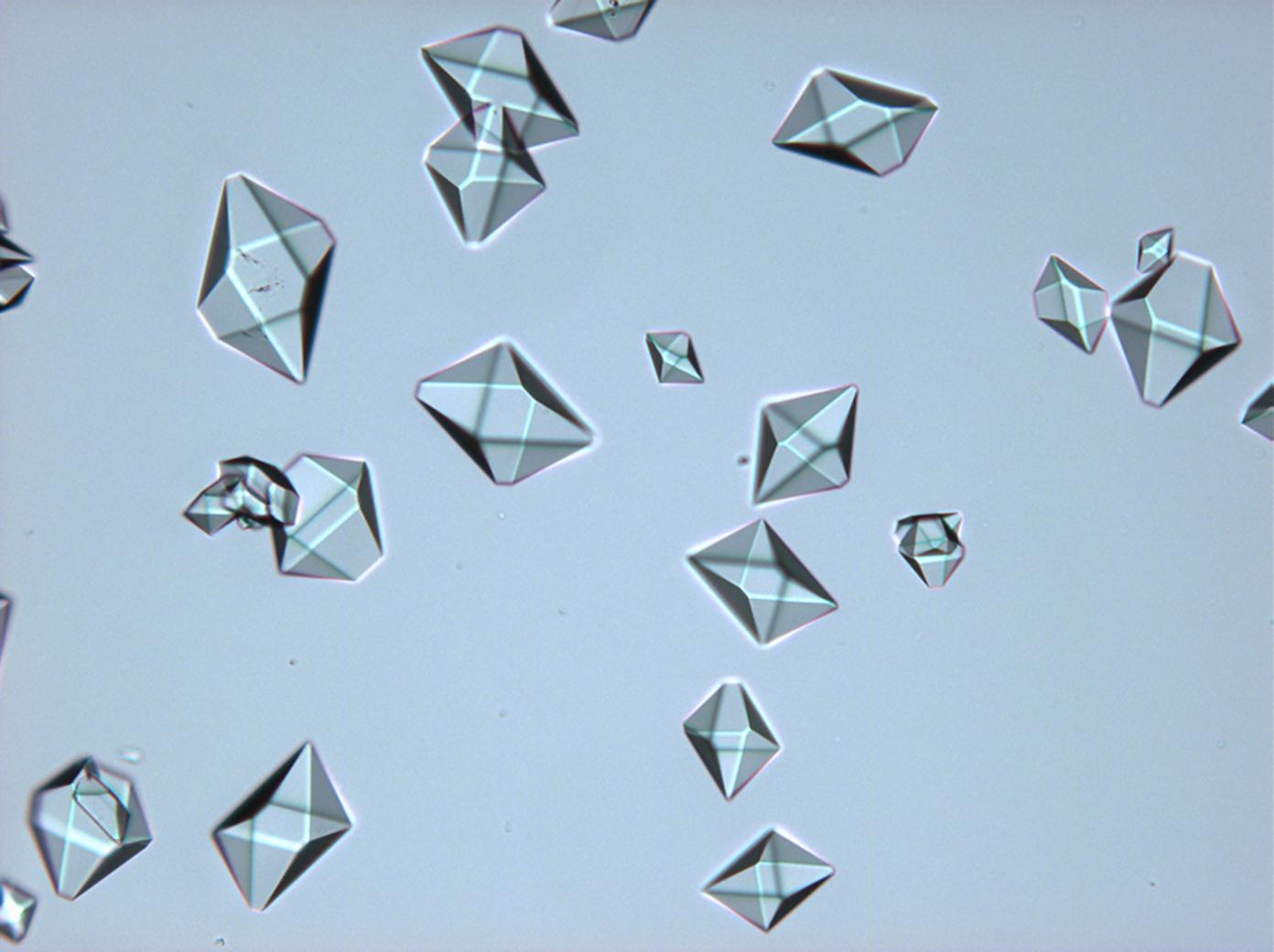
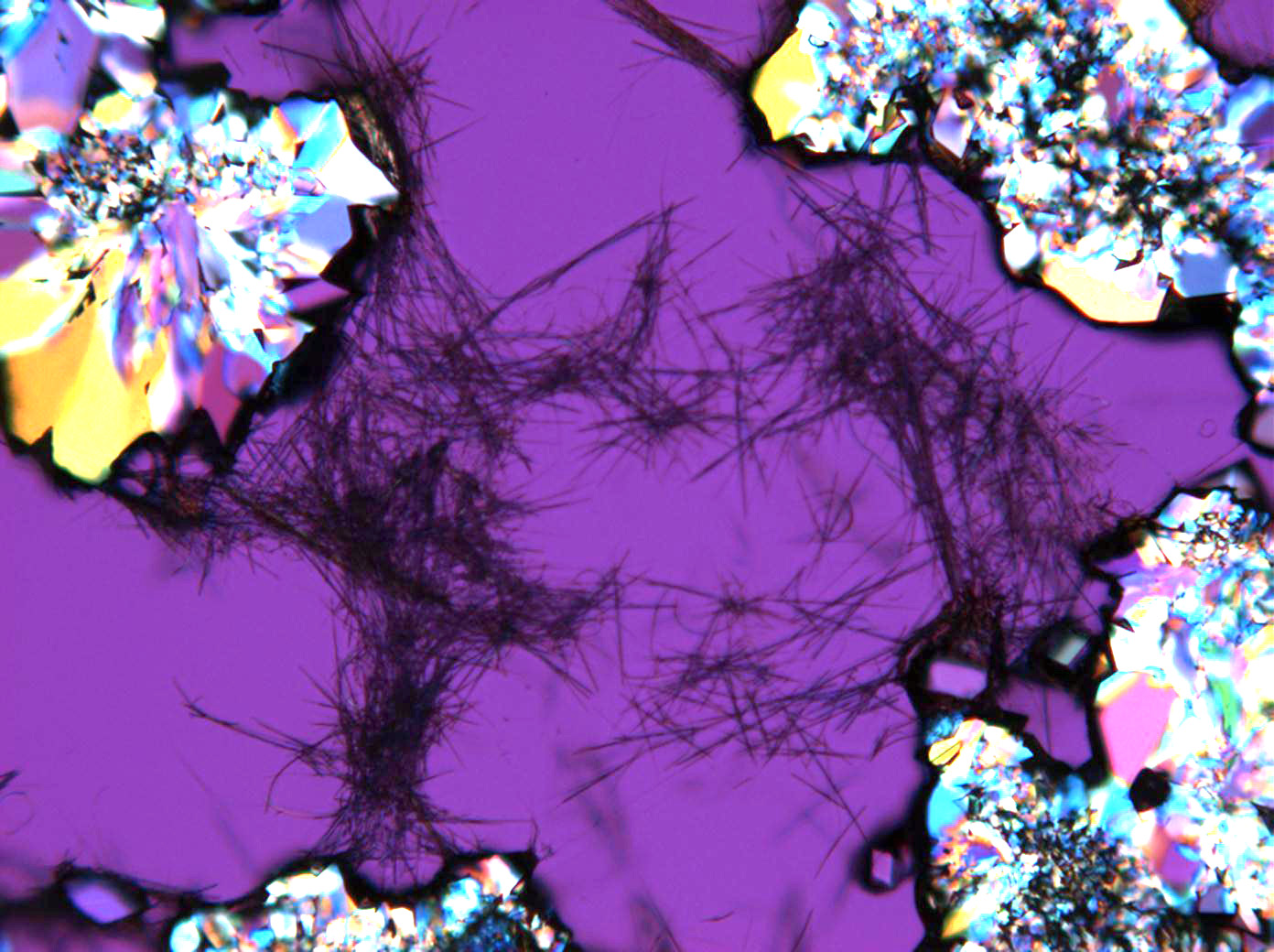
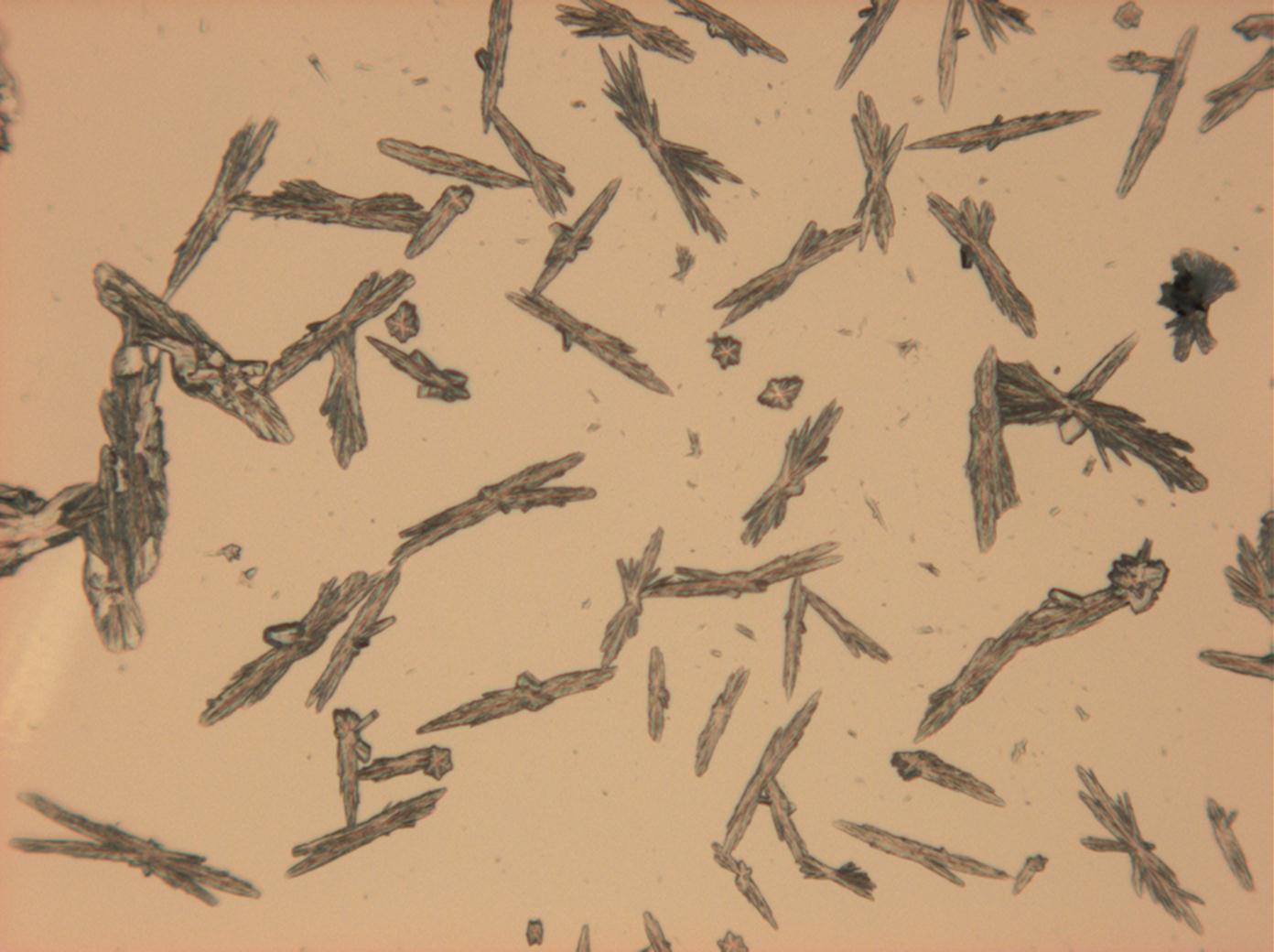
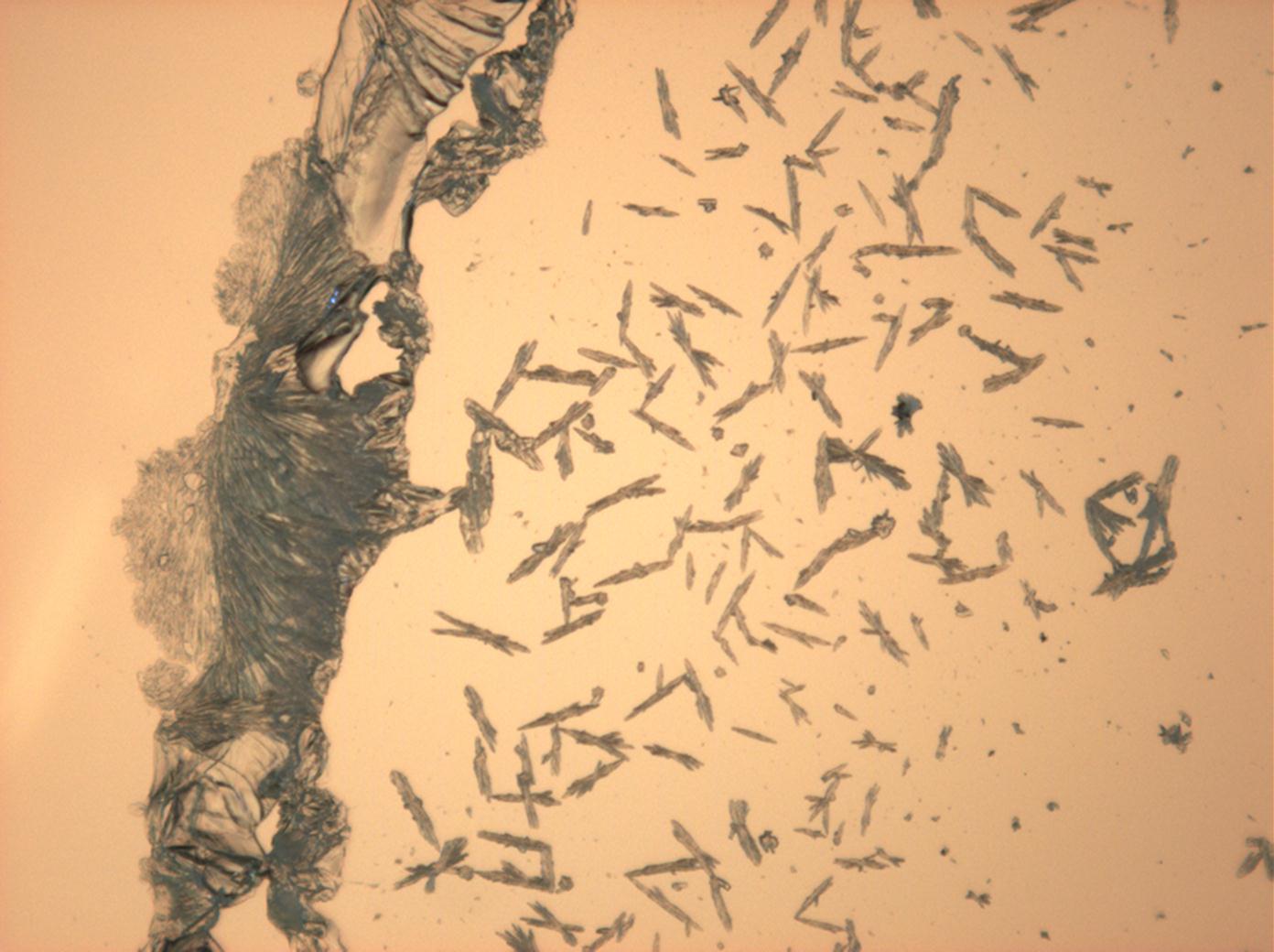
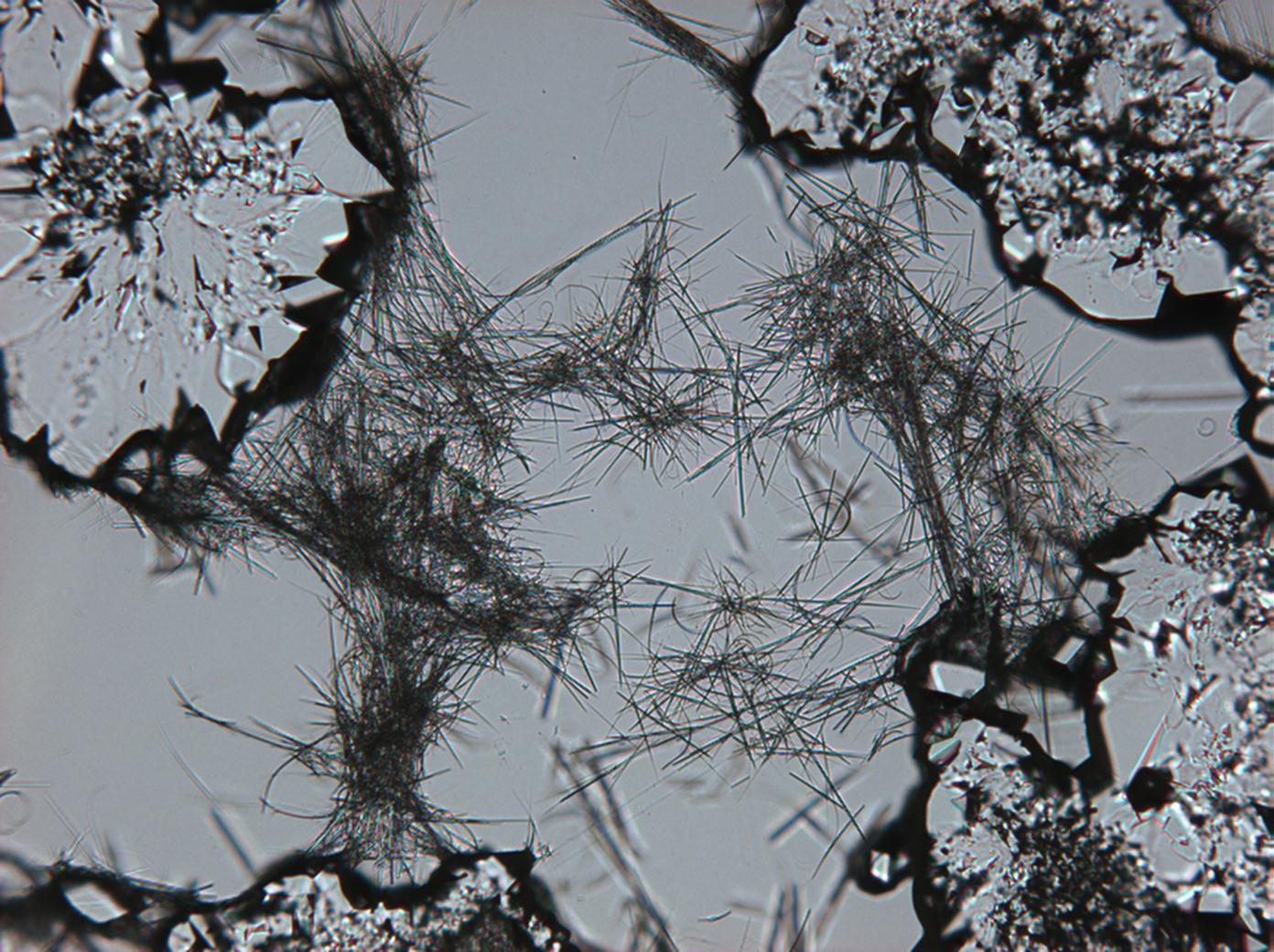
Under the polarizing microscope[edit]
- Sodium sulfate crystals between to glass plates
- Sodium sulfate crystals, crystallised out of a water extraction of a real sample
Weblinks
[edit]
- ↑ http://webmineral.com/data/Thenardite.shtml viewed on 29/07/2010
- ↑ http://www.mindat.org/min-3935.html viewed on 29/07/2010
Literature[edit]
There were no citations found in the article. SLeithaeuser 15:22, 6 May 2012 (CEST)