Nitrocalcite: Difference between revisions
Jump to navigation
Jump to search
Weblinks
| Line 34: | Line 34: | ||
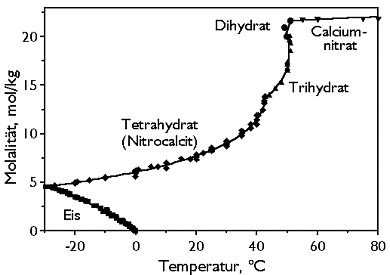
[[file:Nikrocalcit T-Mol-klein.jpg|thumb|400px|left|'''Figure 2''' The system CaNO<sub>3</sub>/H<sub>2</sub>O between -30°C to 0°C (diagram : Michael Steiger)]] | [[file:Nikrocalcit T-Mol-klein.jpg|thumb|400px|left|'''Figure 2''' The system CaNO<sub>3</sub>/H<sub>2</sub>O between -30°C to 0°C (diagram : Michael Steiger)]] | ||
<br clear=all> | <br clear=all> | ||
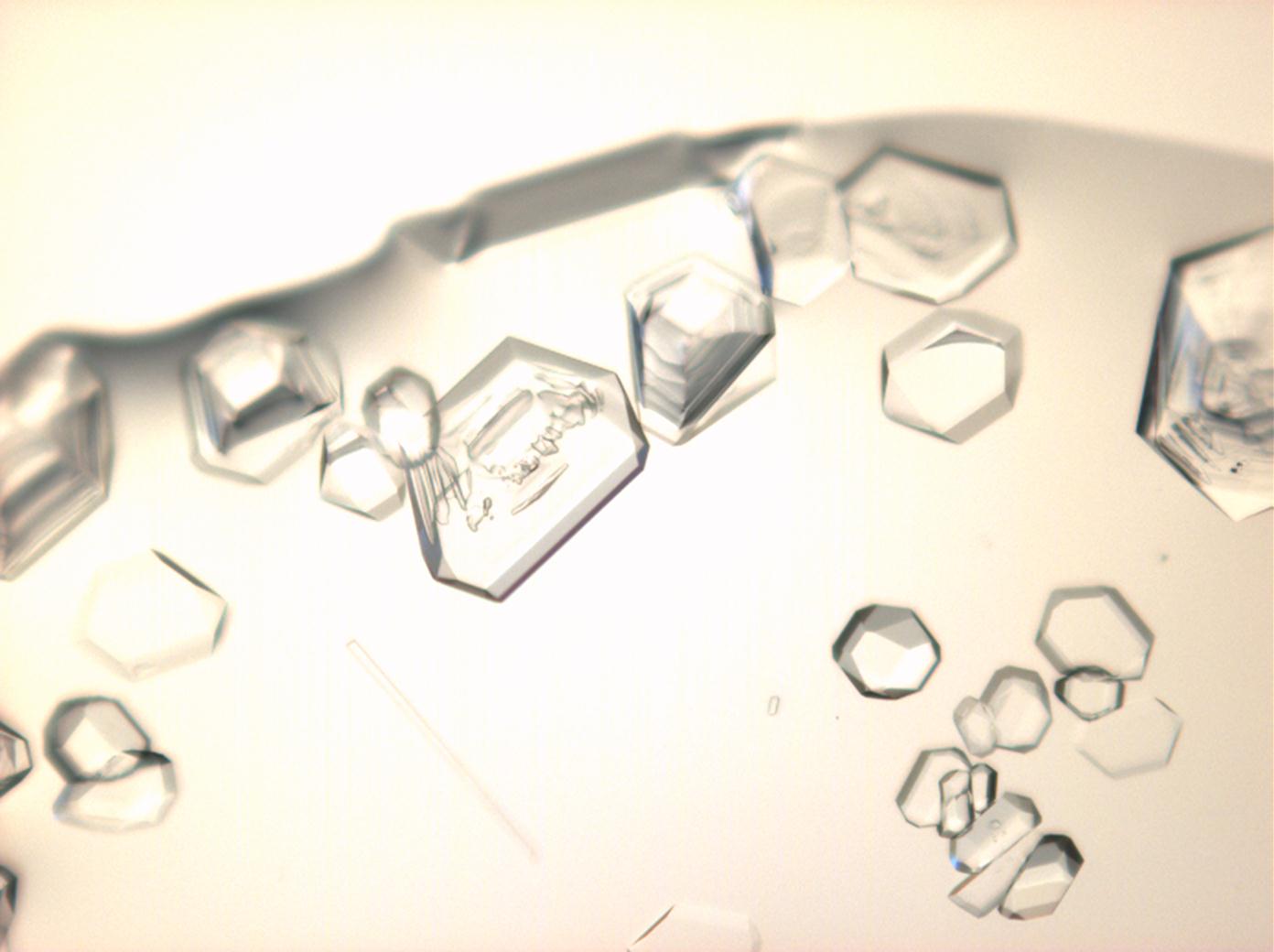
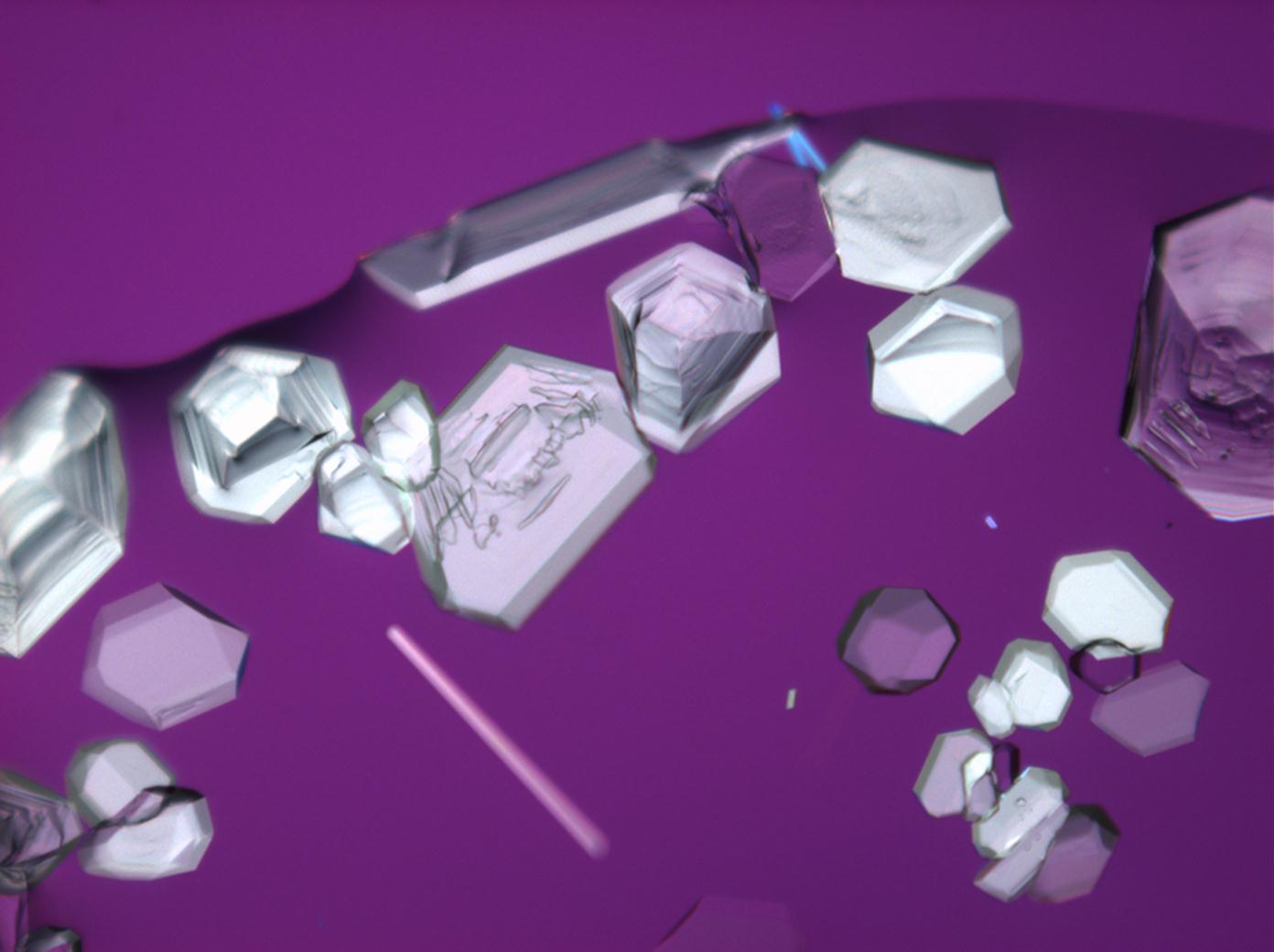
==Under the | ==Under the polarizing microscope == | ||
<gallery caption="Crystallized from a saturated solution with ethanol addition" widths="200px" heights="150px" perrow="3"> | <gallery caption="Crystallized from a saturated solution with ethanol addition" widths="200px" heights="150px" perrow="3"> | ||
Revision as of 15:03, 28 January 2015
| Nitrocalcite[1] | |
| |
| Mineralogical name | Nitrocalcite |
| Chemical name | Calcium Nitrate Tetrahydrate |
| Trivial name | Nitrate of lime |
| Chemical formula | Ca(NO3)2•4H2O |
| Other forms | |
| Crystal system | monoclinic |
| Crystal structure | |
| Deliquescence humidity 20°C | 56.5% (10°C), 53.6% (20°C), 50.5% (25°C) |
| Solubility (g/l) at 20°C | 2660 g/l |
| Density (g/cm³) | 1.82 g/cm3 |
| Molar volume | 129.8 cm3/mol |
| Molar weight | 236.16 g/mol |
| Transparency | transparent |
| Cleavage | |
| Crystal habit | |
| Twinning | |
| Phase transition | |
| Chemical behavior | |
| Comments | |
| Crystal Optics | |
| Refractive Indices | nx = 1.465 ny = 1.498 nz = 1.504 |
| Birefringence | Δ = 0.039 |
| Optical Orientation | negative |
| Pleochroism | |
| Dispersion | |
| Used Literature | |
| {{{Literature}}} | |
back to Nitrate
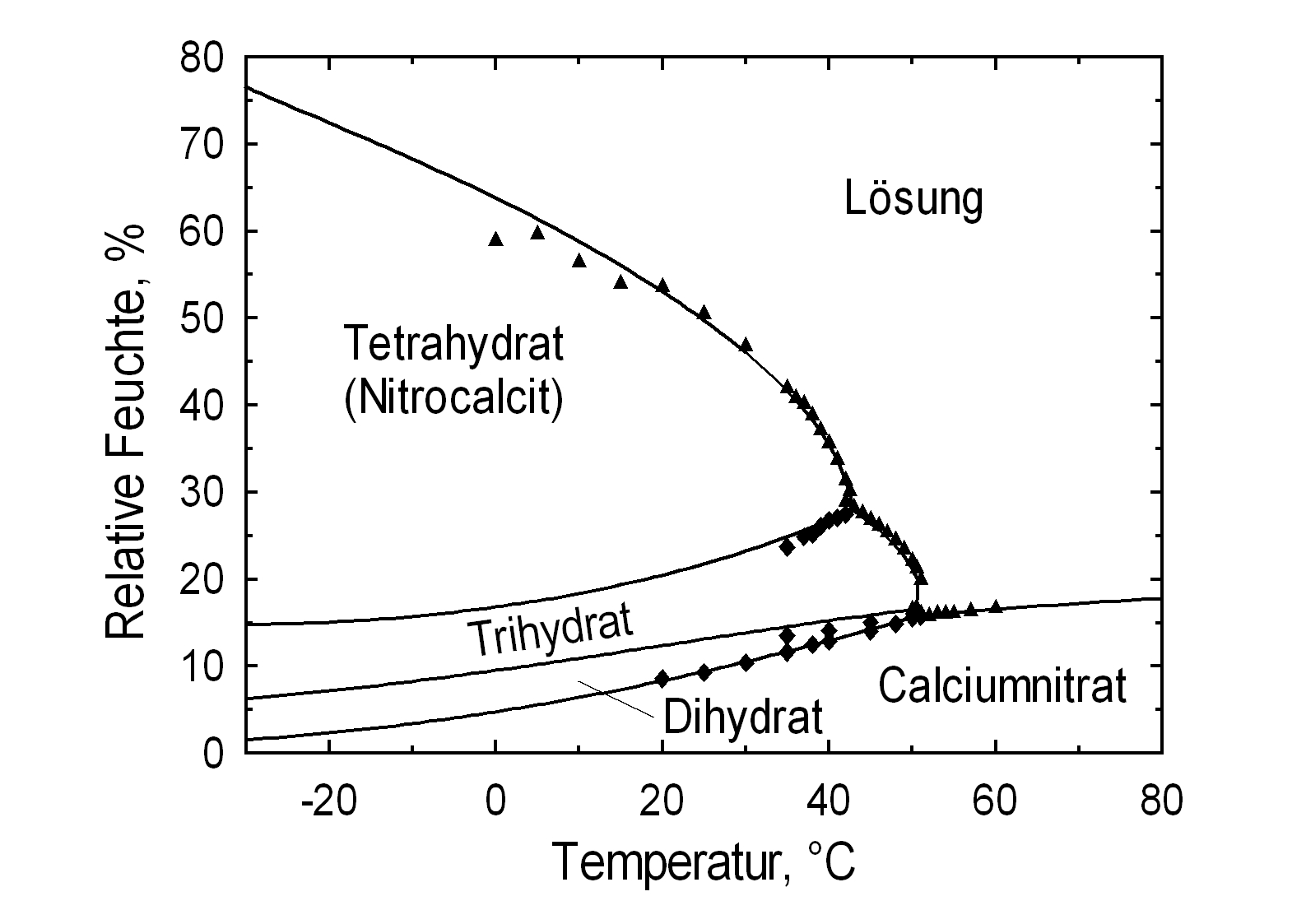
Phase Diagrams[edit]
Under the polarizing microscope[edit]
- Crystallized from a saturated solution with ethanol addition
- Crystallized in a micro climate chamber
Weblinks
[edit]
- ↑ http://www.mindat.org/min-2919.html seen on 29.07.2010