Mirabilite
Jump to navigation
Jump to search
Weblinks
Authors: Hans-Jürgen Schwarz, Nils Mainusch, NN....
English version by Christa Gerdwilker
back to back to Sulfate
| Mirabilite[1][2] | |
| |
| Mineralogical name | Mirabilite |
| Chemical name | Sodium sulfate decahydrate |
| Trivial name | Glauber salt, Reussin, Sulphate of Soda |
| Chemical formula | Na2SO4•10H2O |
| Other forms | Sodium sulphate heptahydrate Na2SO4•7H2O |
| Crystal system | monoclinic |
| Crystal structure | |
| Deliquescence humidity 20°C | 93.6% (20°C), 90% (23°C), 87% (25°C) |
| Solubility (g/l) at 20°C | 900 g/l |
| Density (g/cm³) | 1.464 g/cm³ |
| Molar volume | 219.8 cm3/mol |
| Molar weight | 322.21 g/mol |
| Transparency | transparent to opaque |
| Cleavage | perfect |
| Crystal habit | |
| Twinning | |
| Phase transition | |
| Chemical behavior | |
| Comments | soluble in water and glycerin, not soluble in pure alcohol easily loses some water, converts to thenardite at 32°C abnormal blue or brown interference colors |
| Crystal Optics | |
| Refractive Indices | nx = 1.395 ny = 1.396-1.410 nz = 1.398-1.419 |
| Birefringence | Δ = 0.04-0.023 |
| Optical Orientation | negative |
| Pleochroism | |
| Dispersion | |
| Used Literature | |
| {{{Literature}}} | |
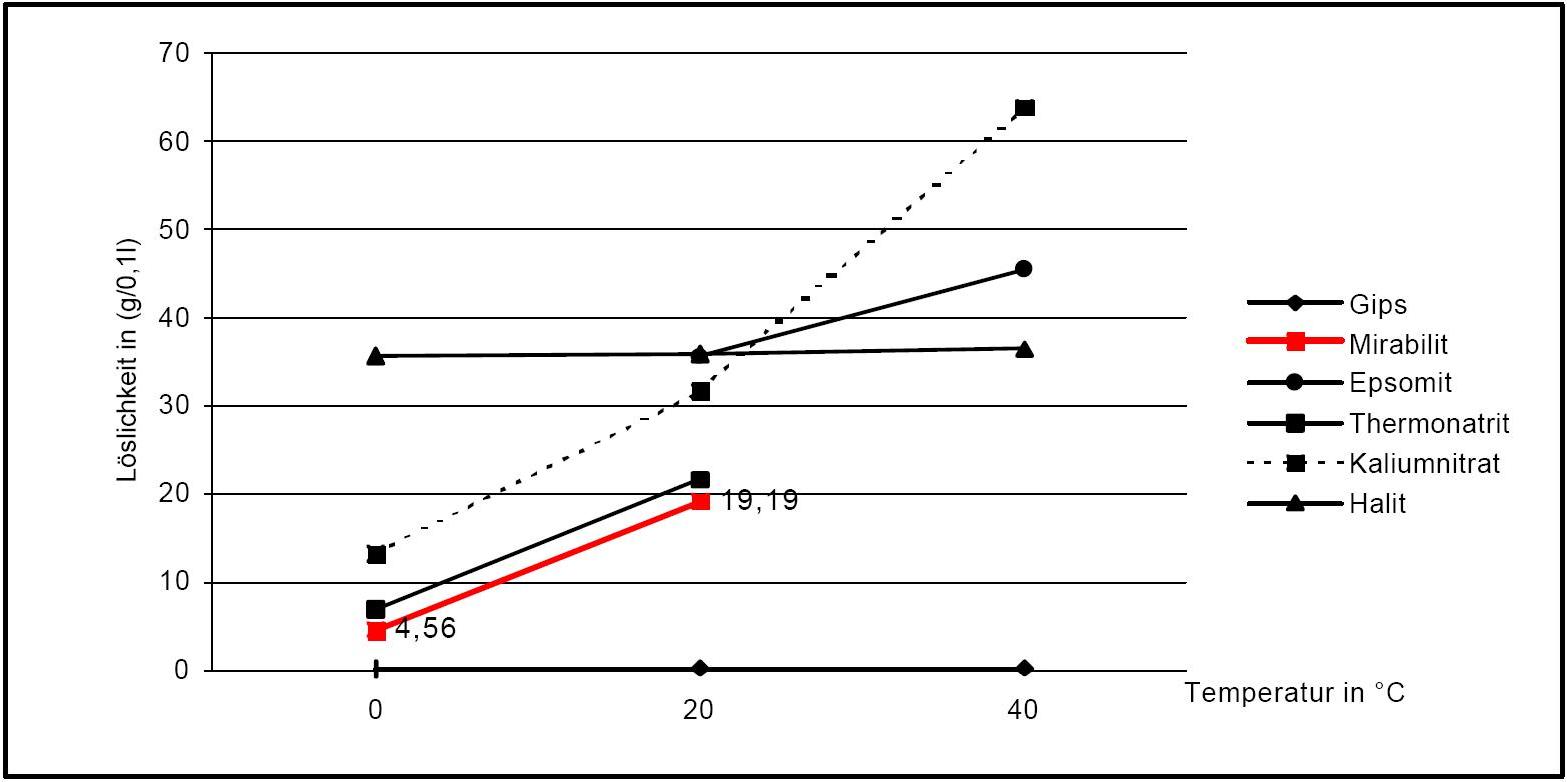
Solubility properties[edit]

Figure1: Solubility of thenardite and mirabilite in comparison to other salts[after [Stark.etal:1996]Title: Bauschädliche Salze
Author: Stark, Jochen; Stürmer, Sylvia
 ].
].
Author: Stark, Jochen; Stürmer, Sylvia
 ].
].
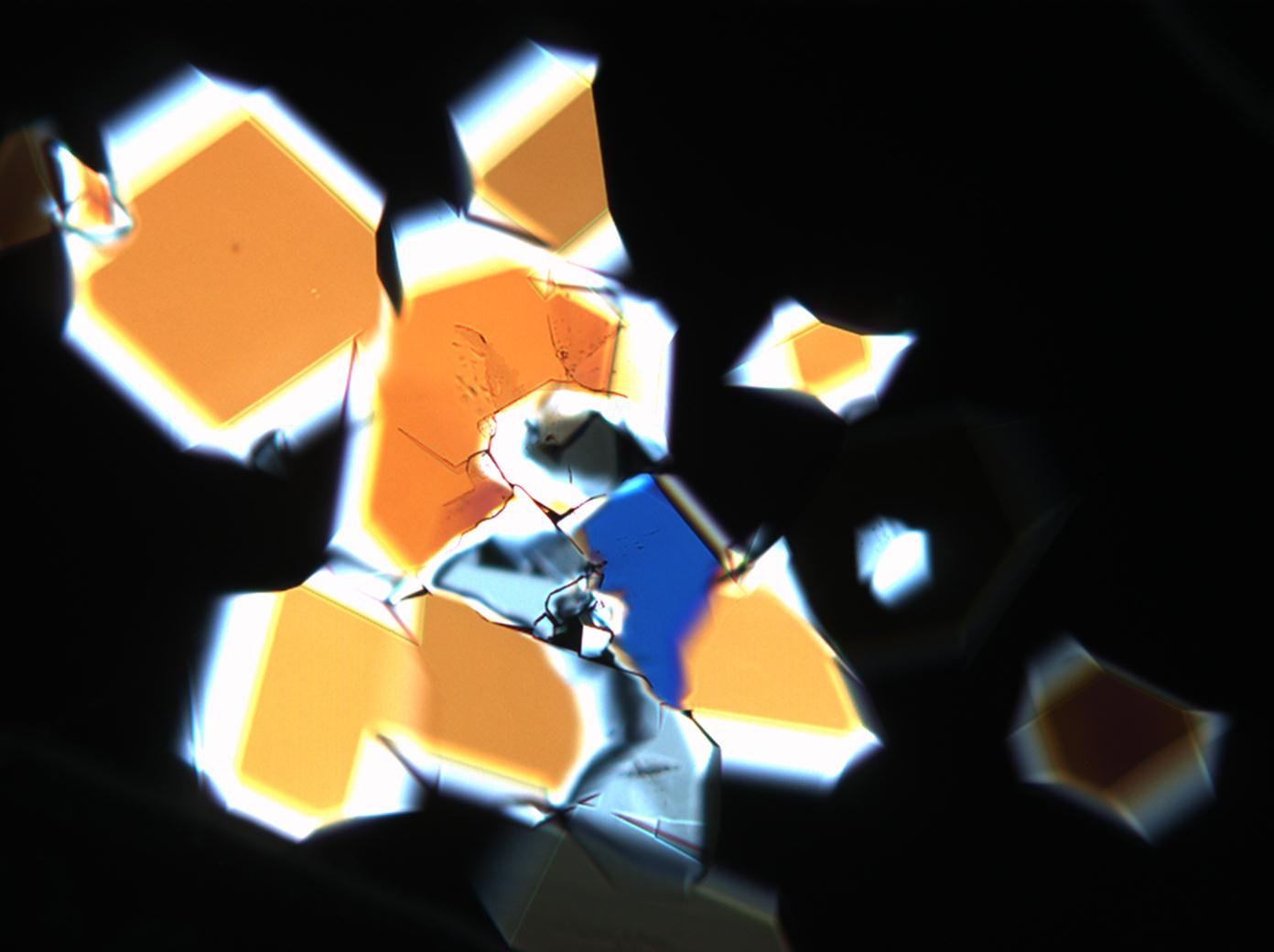
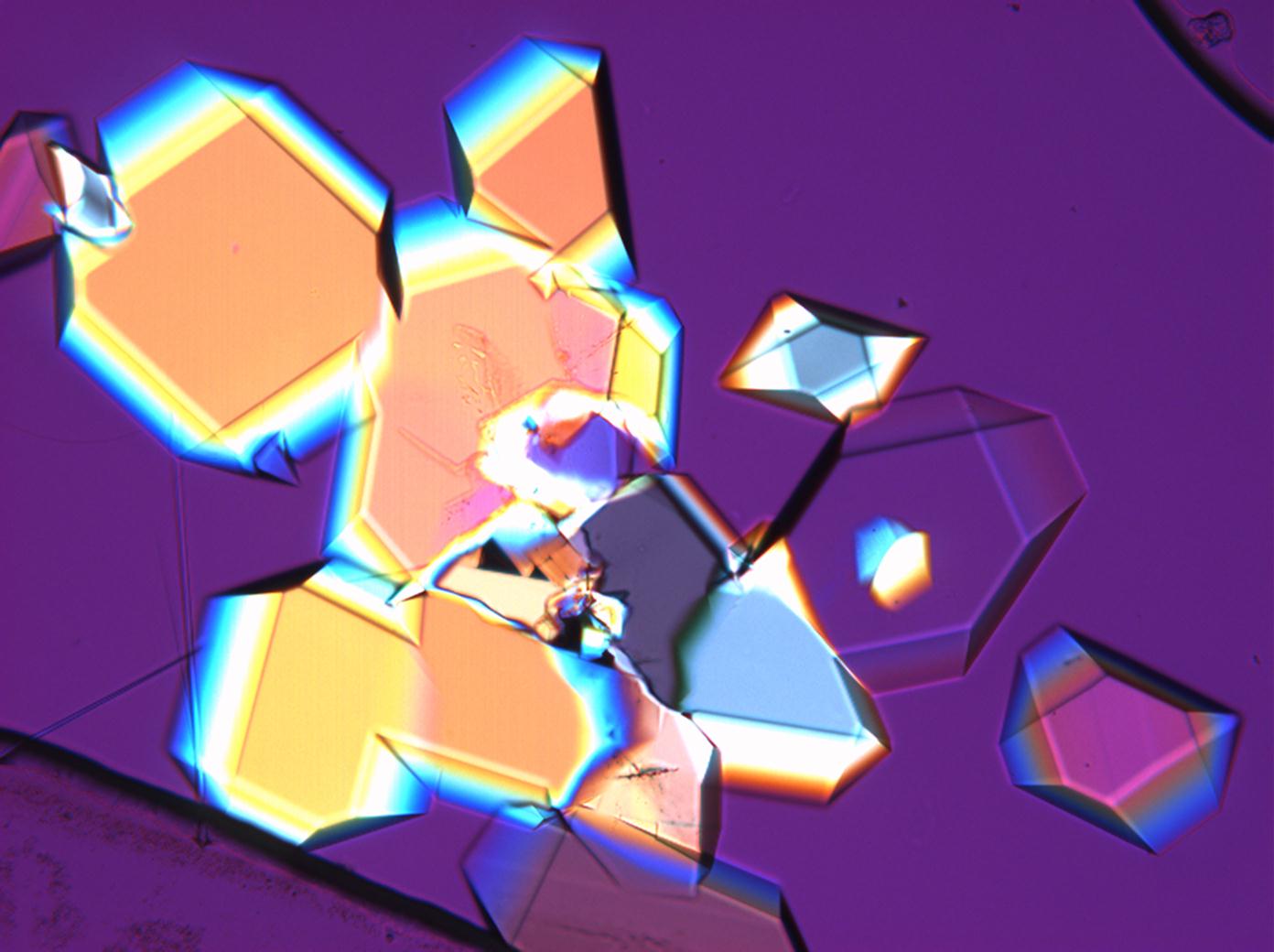
Under the polarizing microscope[edit]
- Sodium sulfate crystallization between two glass slides
Weblinks
[edit]
- ↑ http://webmineral.com/data/Mirabilite.shtml accessed 29/07/2010
- ↑ http://www.mindat.org/min-2725.html accessed 29/07/2010
Literatur[edit]
| [Dana:1951] | Dana E.S. (eds.) Dana J.D. (1951): Dana's System of Mineralogy, 7, Wiley & Sons |  |
| [Robie.etal:1978] | Robie R.A., Hemingway B.S.; Fisher J.A. (1978): Thermodynamic properties of minerals and related substances at 298.15 K and 1 bar pressure and higher temperatures. In: U.S. Geol. Surv. Bull, 1452 () |  |
| [Steiger.etal:2008] | Steiger, Michael; Asmussen, Sönke (2008): Crystallization of sodium sulfate phases in porous materials: The phase diagram Na2SO4–H2O and the generation of stress. In: Geochimica et Cosmochimica Acta, 72 (17), 4291-4306, Url |  |
| [Vogt.etal:1993] | Vogt, R.; Goretzki, Lothar (1993): Der Einfluss hygroskopischer Salze auf die Gleichgewichtsfeuchte und Trocknung anorganischer Baustoffe, unveröffentlichter Bericht. |  |