Aphthitalite: Difference between revisions
Jump to navigation
Jump to search
No edit summary |
No edit summary |
||
| (9 intermediate revisions by 3 users not shown) | |||
| Line 11: | Line 11: | ||
|Deliqueszenzhumidity = | |Deliqueszenzhumidity = | ||
|Solubility = | |Solubility = | ||
|Density = | |Density = 1.35 g/cm<sup>3</sup> | ||
|MolVolume = 246.2 cm<sup>3</sup>/mol | |MolVolume = 246.2 cm<sup>3</sup>/mol | ||
|Molweight = 332 g/mol | |Molweight = 332.41 g/mol | ||
|Transparency = transparent to opaque | |Transparency = transparent to opaque | ||
|Cleavage = imperfect to fair | |Cleavage = imperfect to fair | ||
|Crystal_Habit = | |Crystal_Habit = | ||
|Twinning = | |Twinning = | ||
|Refractive_Indices = n<sub>o</sub> = | |Refractive_Indices = n<sub>o</sub> = 1.491<br> n<sub>e</sub> = 1.499 | ||
|Birefringence = Δ = | |Birefringence = Δ = 0.008 | ||
|optical_Orientation = positive | |optical_Orientation = positive | ||
|Pleochroism = | |Pleochroism = | ||
| Line 26: | Line 26: | ||
|chemBehavior = | |chemBehavior = | ||
|Comments = | |Comments = | ||
|Literature = <bib id="JCPDS:1991"/><bib id="Dana:1951"/> | |||
}} | }} | ||
back to [[Sulfate]] | back to [[Sulfate]] | ||
== | ==References== | ||
<references/> | <references/> | ||
[[Category:Aphthitalite]][[Category:Sulphate]][[Category:Salt]][[Category: | == Literature == | ||
<biblist/> | |||
[[Category:Aphthitalite]][[Category:Sulfate]][[Category:Sulphate]][[Category:Salt]][[Category:Editing]][[Category:List]] | |||
Latest revision as of 16:49, 14 February 2015
| Aphthitalite[1][2] | |
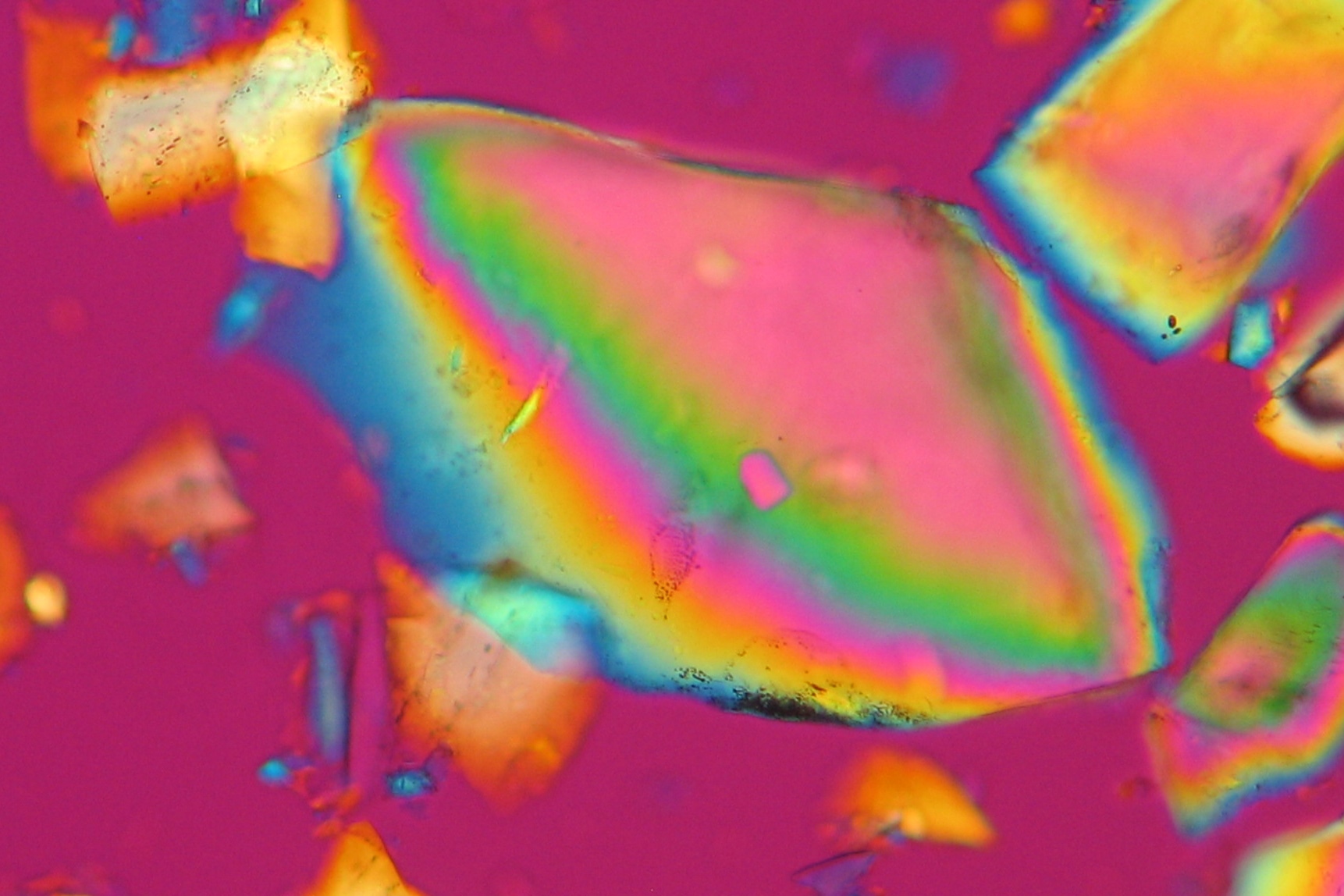
| |
| Mineralogical name | Aphthitalite |
| Chemical name | Potassium sodium sulphate |
| Trivial name | Glaserite, Vesuvian Salt |
| Chemical formula | K3Na(SO4)2 Na2SO4•3K2SO4 |
| Other forms | |
| Crystal system | trigonal |
| Crystal structure | |
| Deliquescence humidity 20°C | |
| Solubility (g/l) at 20°C | |
| Density (g/cm³) | 1.35 g/cm3 |
| Molar volume | 246.2 cm3/mol |
| Molar weight | 332.41 g/mol |
| Transparency | transparent to opaque |
| Cleavage | imperfect to fair |
| Crystal habit | |
| Twinning | |
| Phase transition | |
| Chemical behavior | |
| Comments | |
| Crystal Optics | |
| Refractive Indices | no = 1.491 ne = 1.499 |
| Birefringence | Δ = 0.008 |
| Optical Orientation | positive |
| Pleochroism | |
| Dispersion | |
| Used Literature | |
[JCPDS:1991]Title: JCPDS Powder Diffraction File 2 (PDF-2) [Dana:1951]Title: Dana's System of Mineralogy [Dana:1951]Title: Dana's System of MineralogyAuthor: Dana J.D. 
| |
back to Sulfate
References[edit]
- ↑ http://webmineral.com/data/Aphthitalite.shtml viewed on 29/07/2010
- ↑ http://www.mindat.org/min-280.html viewed on 29/07/2010
Literature[edit]
| [Dana:1951] | Dana E.S. (eds.) Dana J.D. (1951): Dana's System of Mineralogy, 7, Wiley & Sons |  |
| [JCPDS:1991] | (1991): JCPDS Powder Diffraction File 2 (PDF-2). |  |