Epsomite: Difference between revisions
Jump to navigation
Jump to search
| Line 200: | Line 200: | ||
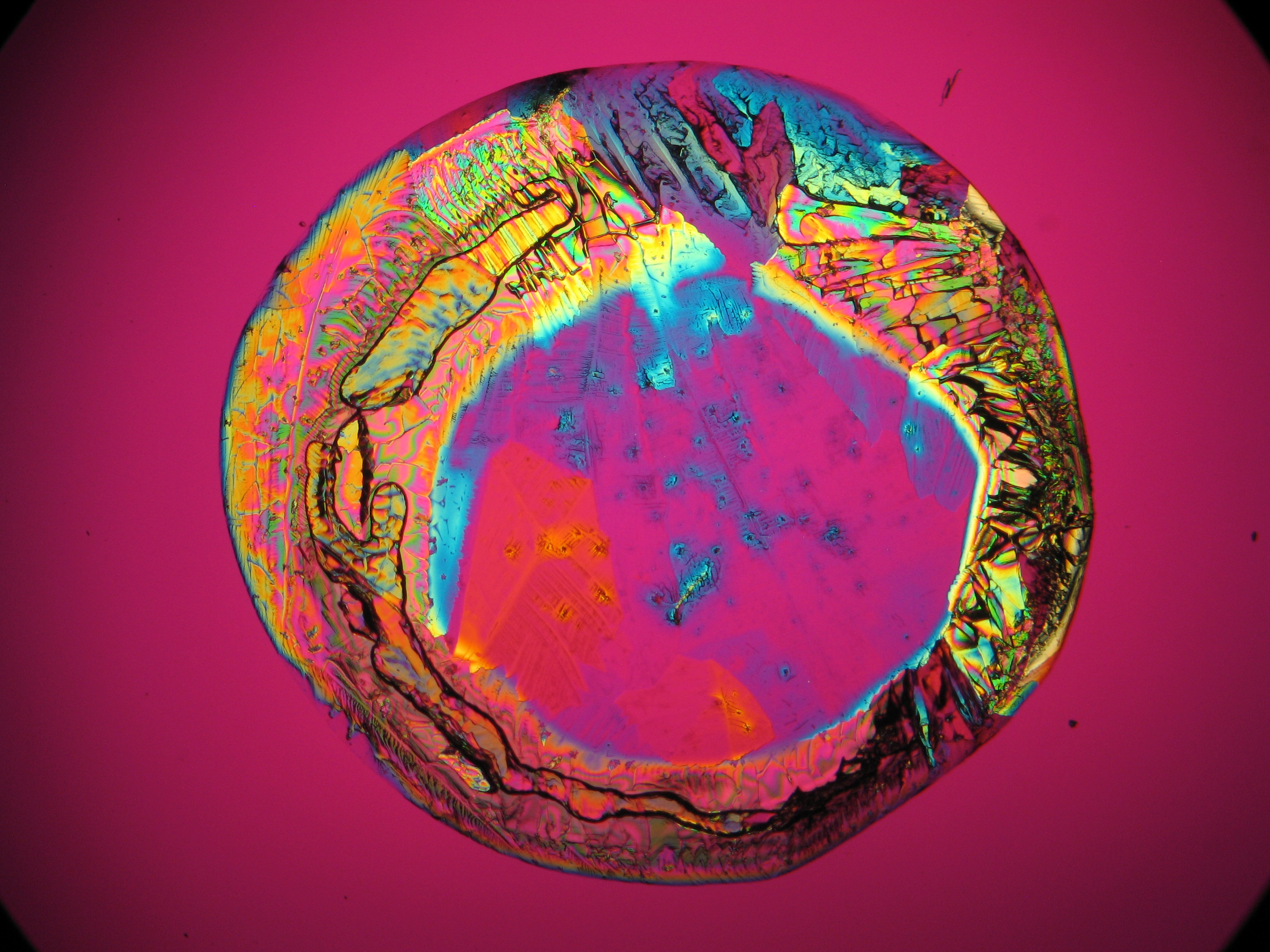
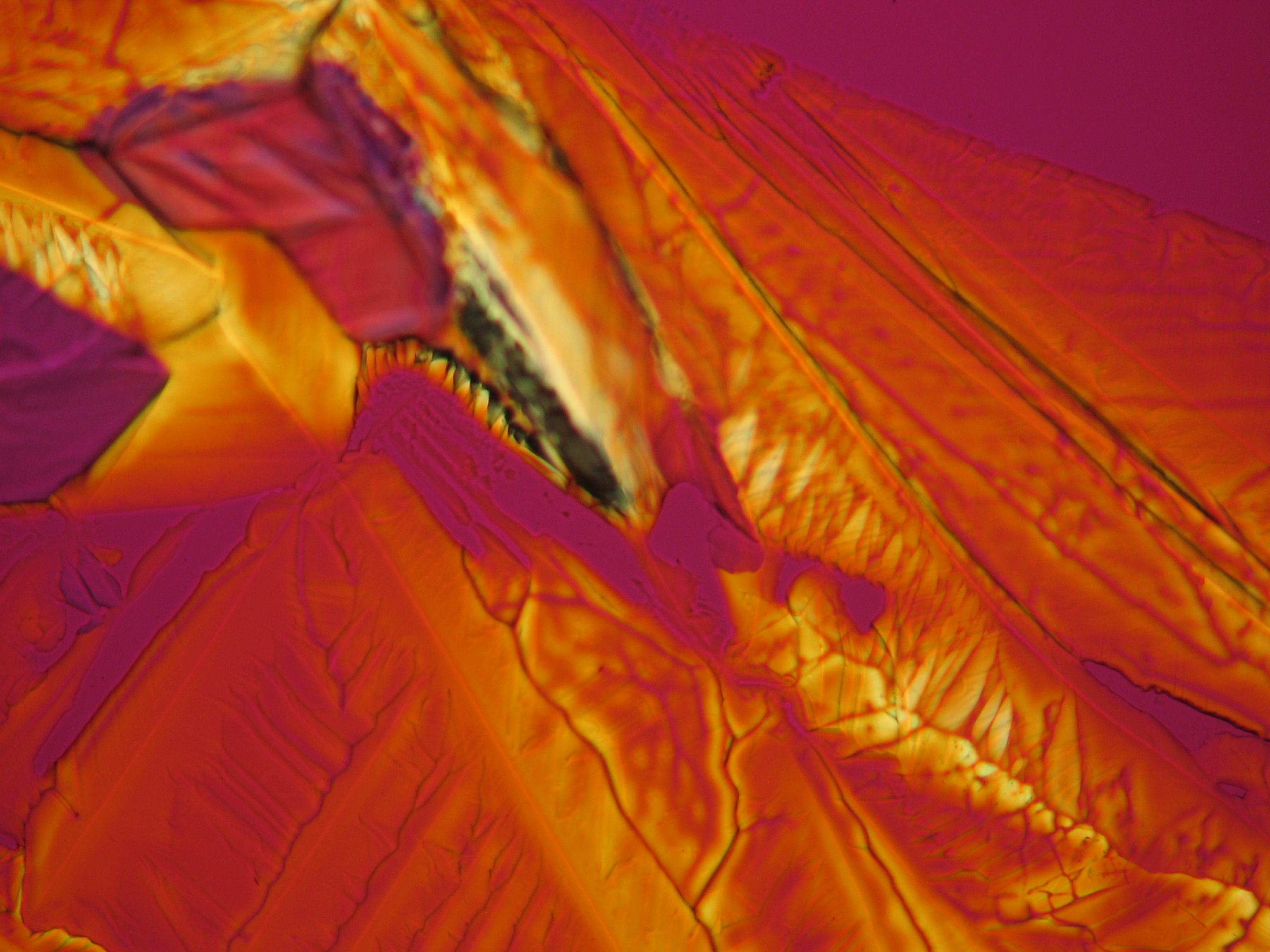
Image:CeS 221209-04-2,5kx-02.JPG|Real sample with magnesium sulfate salts, crystallized from a water solution on a glass slide | Image:CeS 221209-04-2,5kx-02.JPG|Real sample with magnesium sulfate salts, crystallized from a water solution on a glass slide | ||
Image:CeS 221209-04-2,5kx-03.JPG|Real sample with magnesium sulfate salts, crystallized from a water solution on a glass slide | Image:CeS 221209-04-2,5kx-03.JPG|Real sample with magnesium sulfate salts, crystallized from a water solution on a glass slide | ||
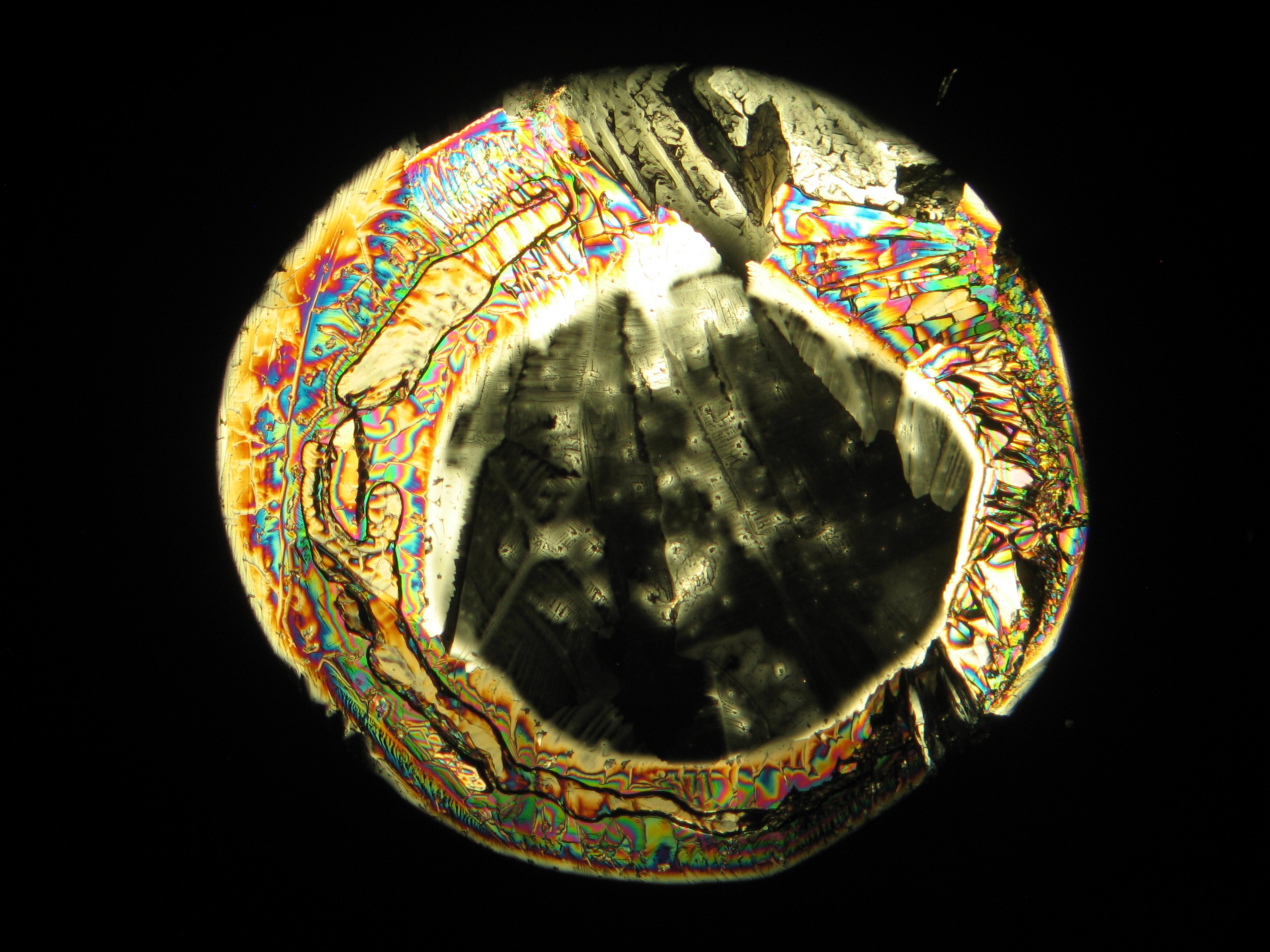
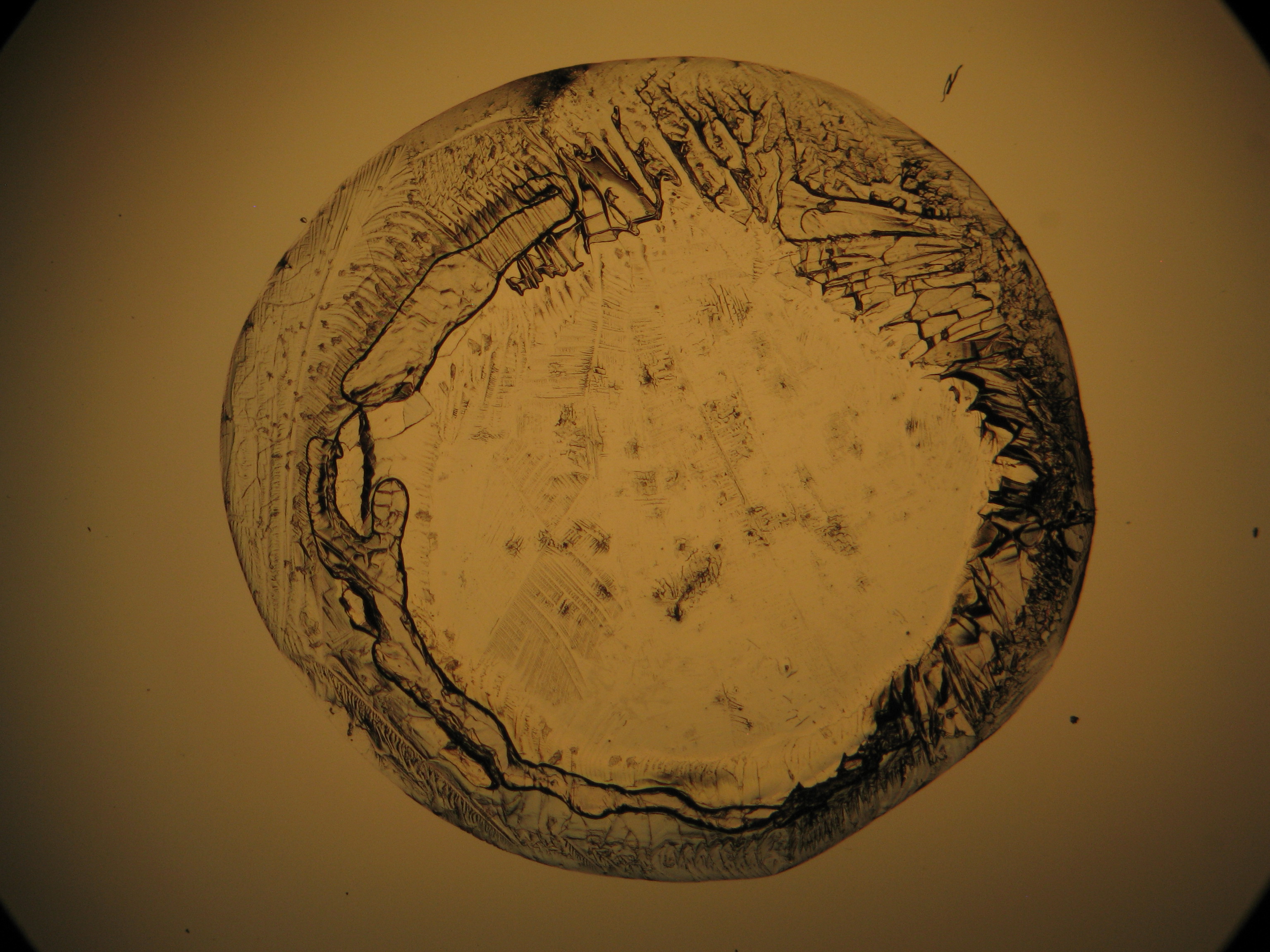
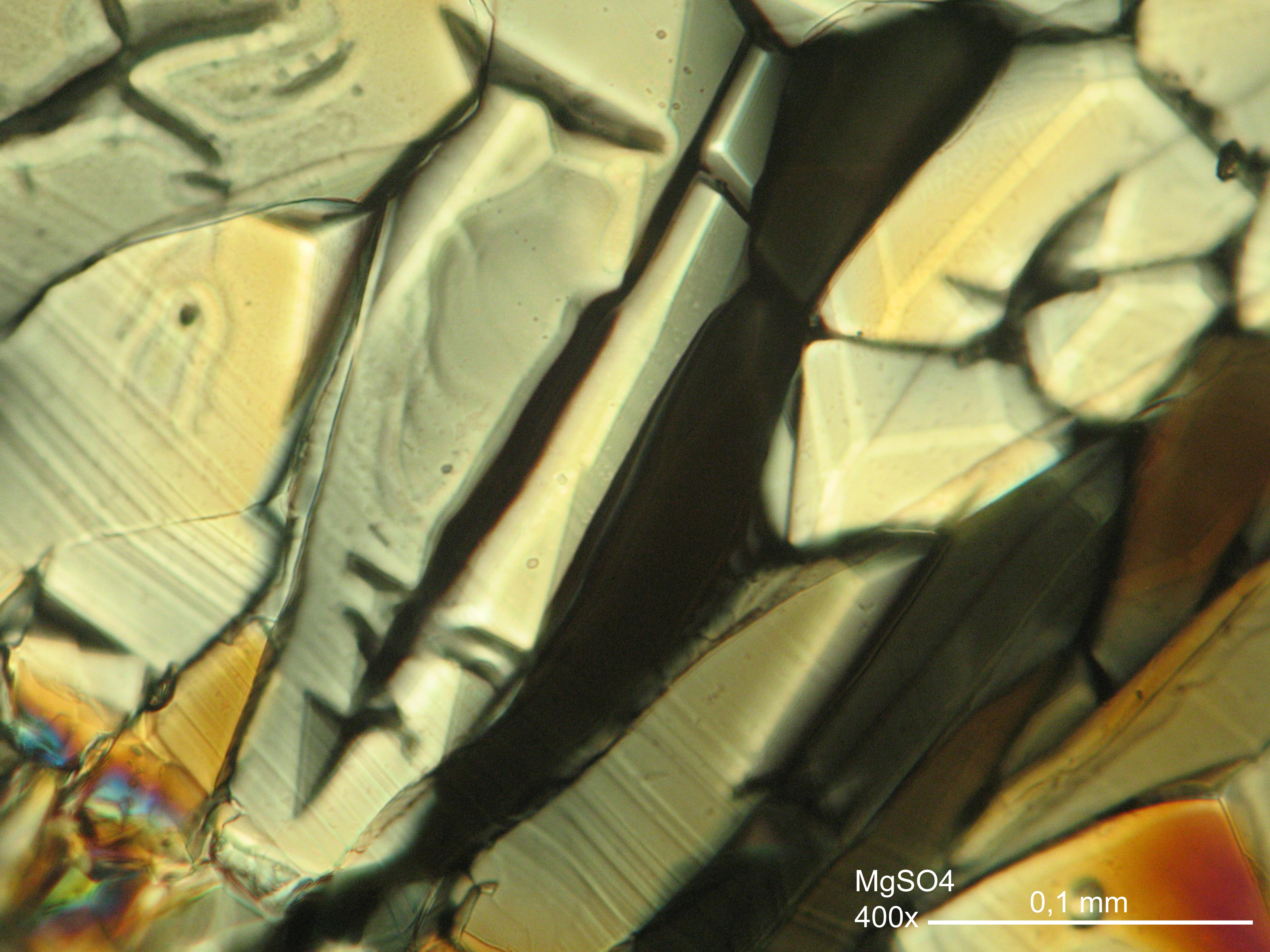
Image:MGSO4 4.5.2006 (11).JPG|Magnesium- ulfate, crystallized from a water solution on a glass slide | Image:MGSO4 4.5.2006 (11).JPG|Magnesium- ulfate, crystallized from a water solution on a glass slide | ||
Image:MGSO4 4.5.2006 (12).JPG|Magnesium sulfate, crystallized from a water solution on a glass slide | Image:MGSO4 4.5.2006 (12).JPG|Magnesium sulfate, crystallized from a water solution on a glass slide | ||
Revision as of 15:59, 26 December 2011
| Epsomite[1][2] | |
| |
| Mineralogical name | Epsomite |
| Chemical name | Magnesiumsulfate Heptahydrate |
| Trivial name | Bitter Salts, Reichardtite, Seelandite |
| Chemical formula | MgSO4•7H2O |
| Other forms | Kieserite (MgSO4•H2O) Sanderite (MgSO4•2H2O) Starkeyite (MgSO4•4H2O) Pentahydrite(MgSO4•5H2O) Hexahydrite (MgSO4•6H2O) Meridianite (MgSO4•11H2O) Magnesium 12-Hydrate |
| Crystal system | orthorhombic |
| Crystal structure | |
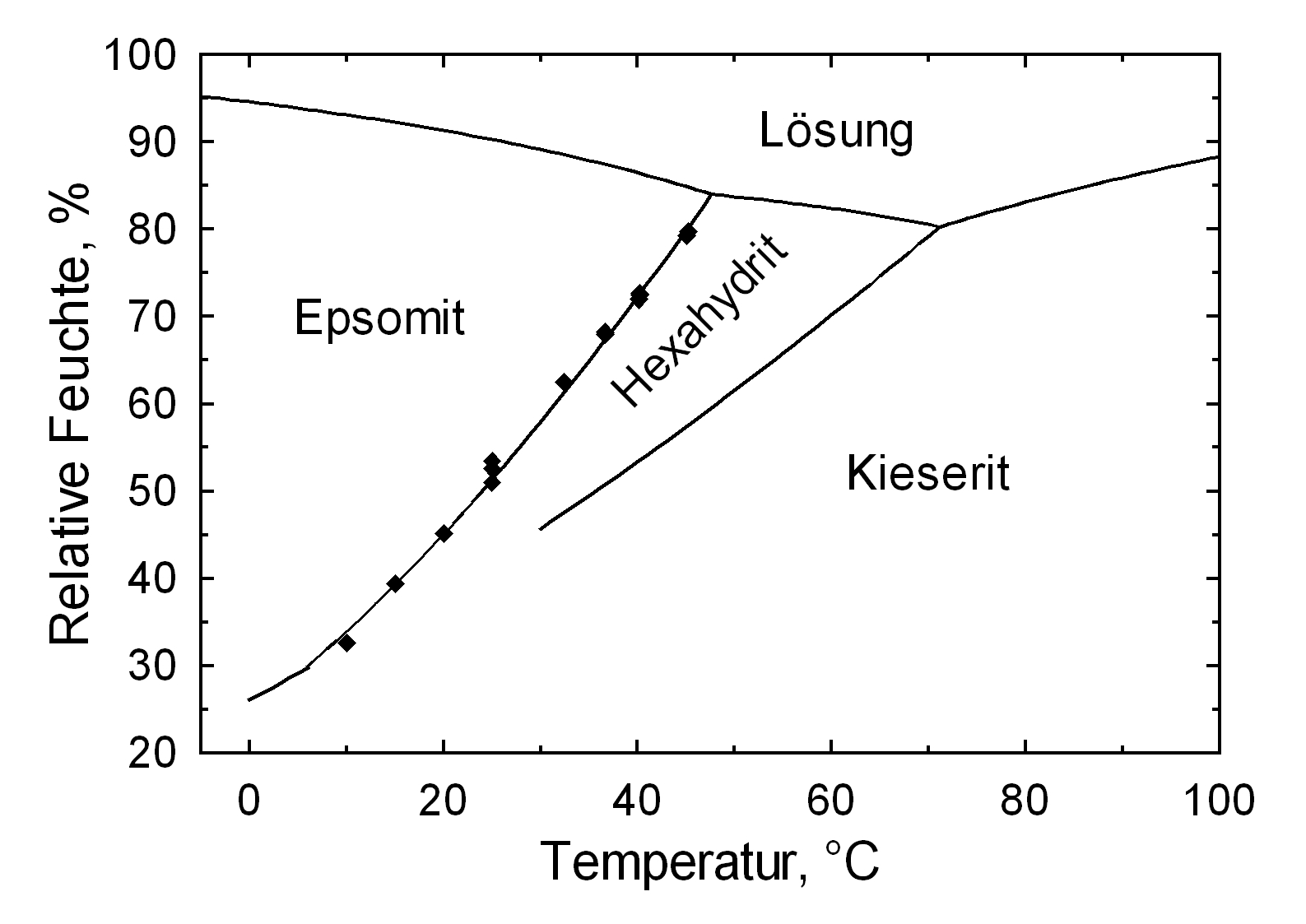
| Deliquescence humidity 20°C | 90.1% (20°C), 94% (30°C) |
| Solubility (g/l) at 20°C | 710 g/l |
| Density (g/cm³) | 146.8 cm3/mol |
| Molar volume | 246.48 g/mol |
| Molar weight | 1.67 g/cm3 |
| Transparency | transparent to translucent |
| Cleavage | perfect |
| Crystal habit | |
| Twinning | |
| Phase transition | |
| Chemical behavior | |
| Comments | can by produced from an aqueous solution under 50°C |
| Crystal Optics | |
| Refractive Indices | nx = 1.433 ny = 1.455 nz = 1.461 |
| Birefringence | Δ = 0.028 |
| Optical Orientation | biaxial negative |
| Pleochroism | |
| Dispersion | |
| Used Literature | |
| {{{Literature}}} | |
<bibimport/>
Authors: Hans-Jürgen Schwarz, Tim Müller, Nils Mainusch
back to Sulfate
Hygroscopicity[edit]
| 10°C | 20°C | 25°C | 30°C |
| 86,9% r.F. | 90,1% r.F. | 88,3% r.F. | 88,0% r.F. |
Sorption of moisture[edit]
| Moisture sorption at | 87%r.F. | 81%r.F. | 70%r.F. | 61%r.F. | 50%r.F. |
| MgSO4 | 76 | 75 | 70 | 71 | 27 |
| MgSO4 + NaCl (1:1 molare mixture) |
240 | 146 | 75 | 50 | 20 |
Salts and deteriation pattern[edit]
On objects[edit]
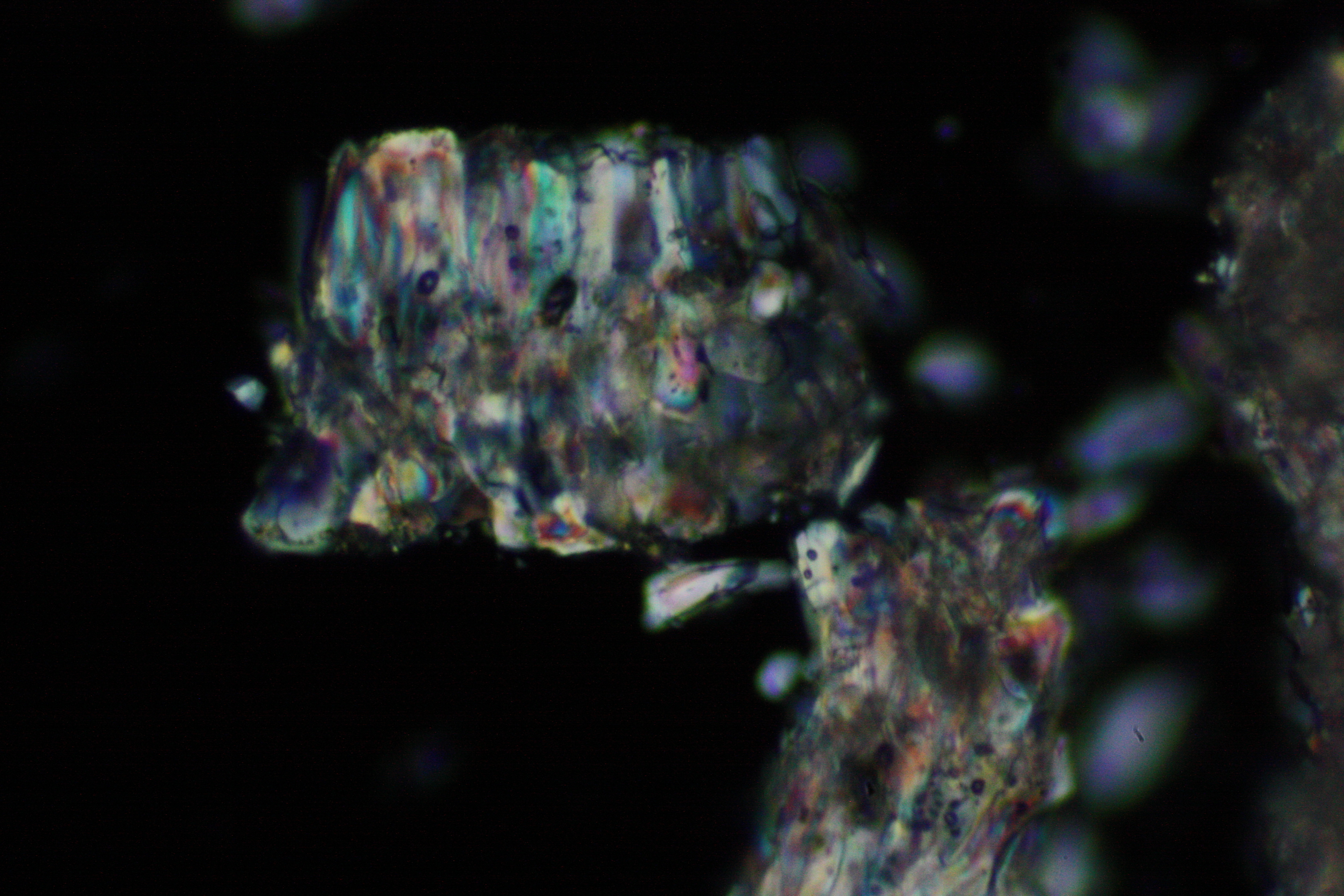
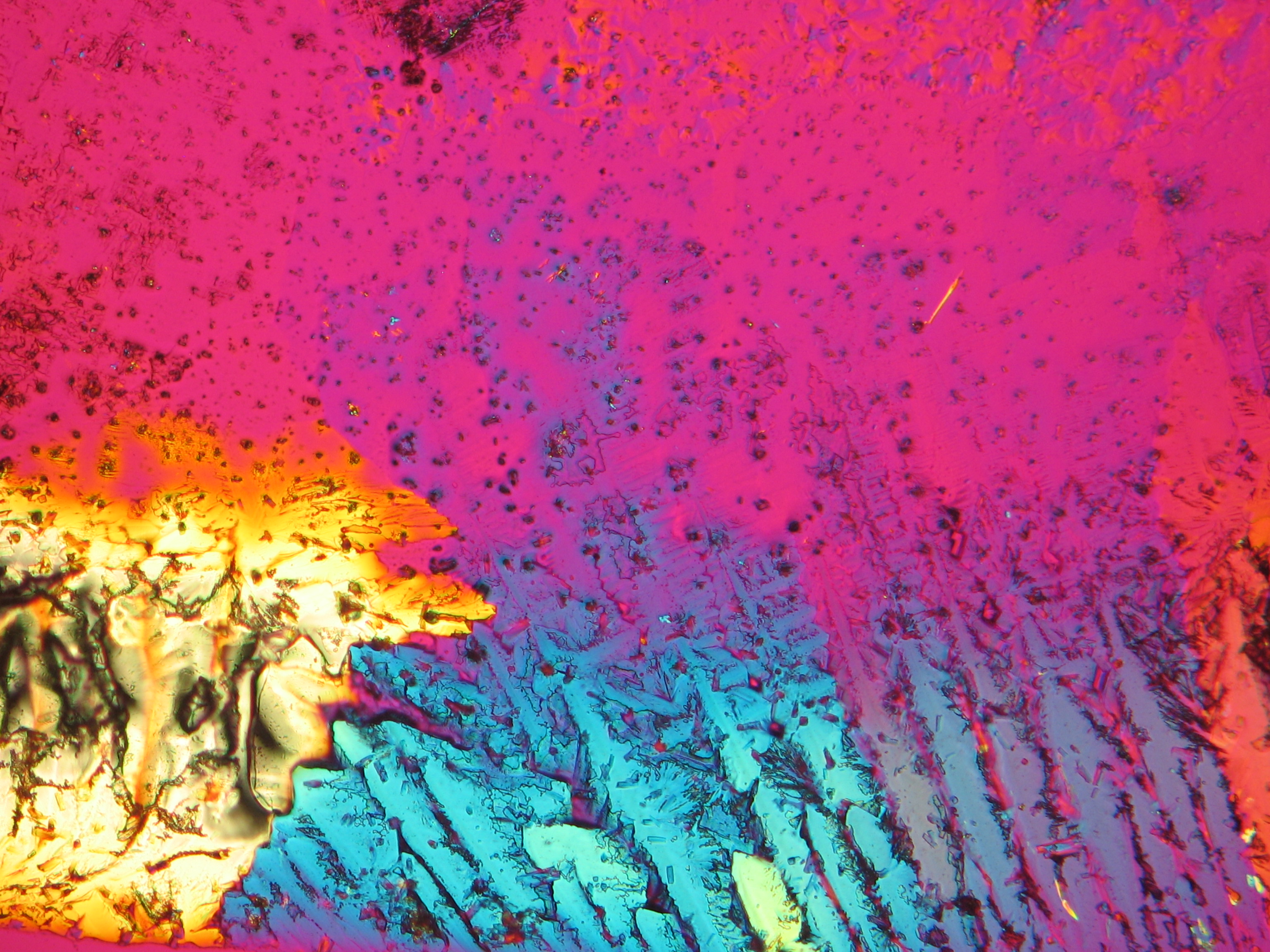
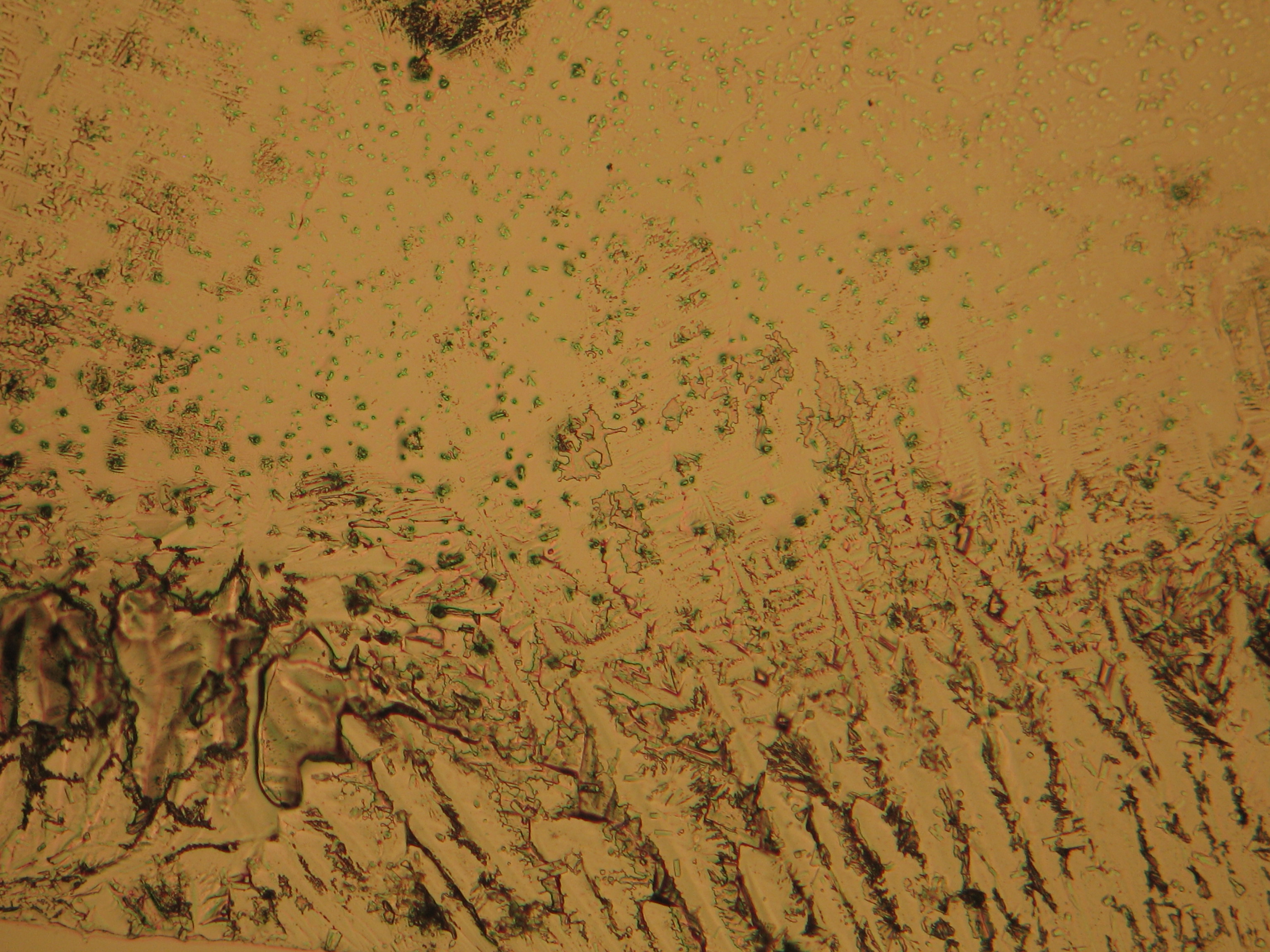
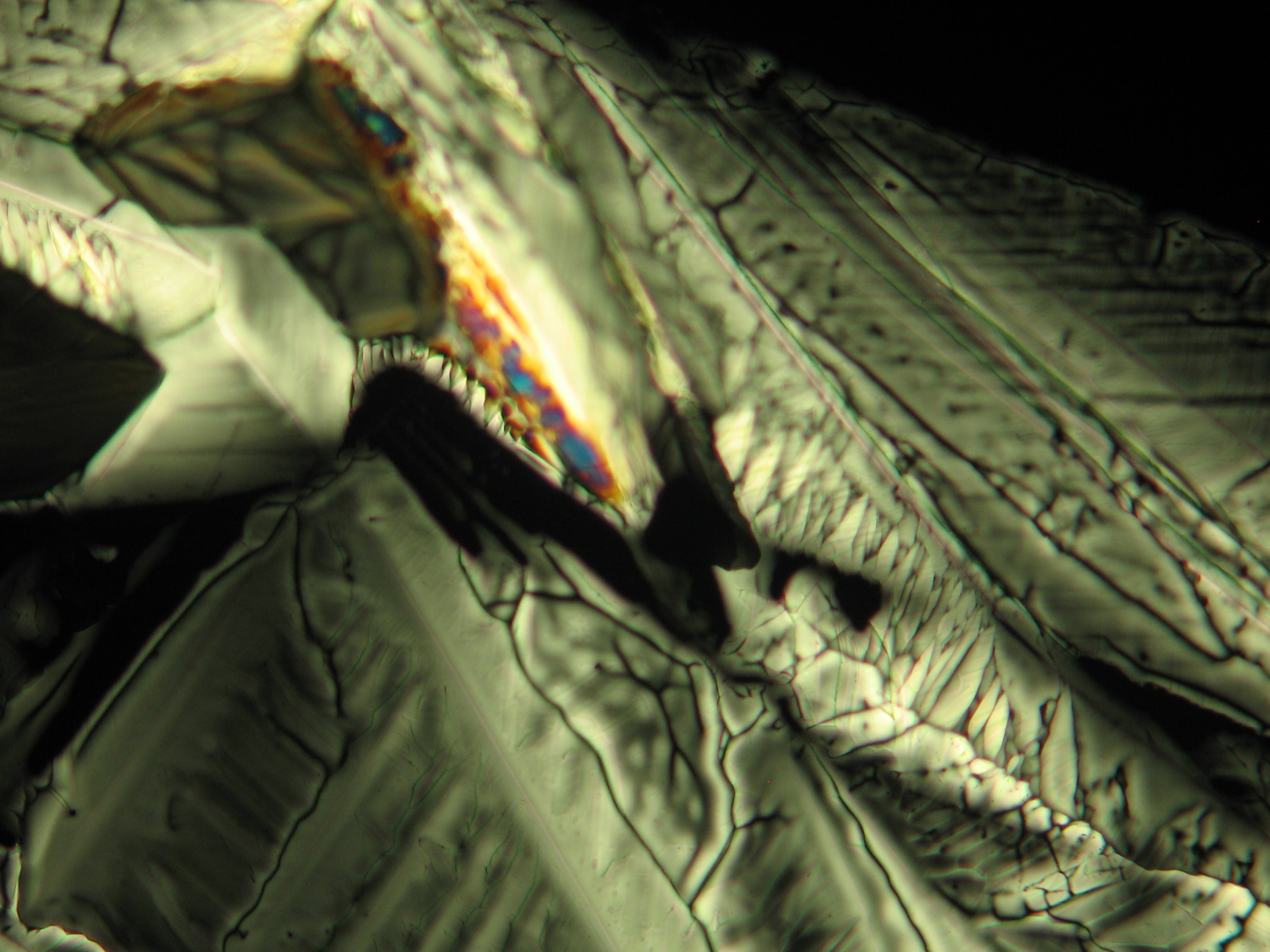
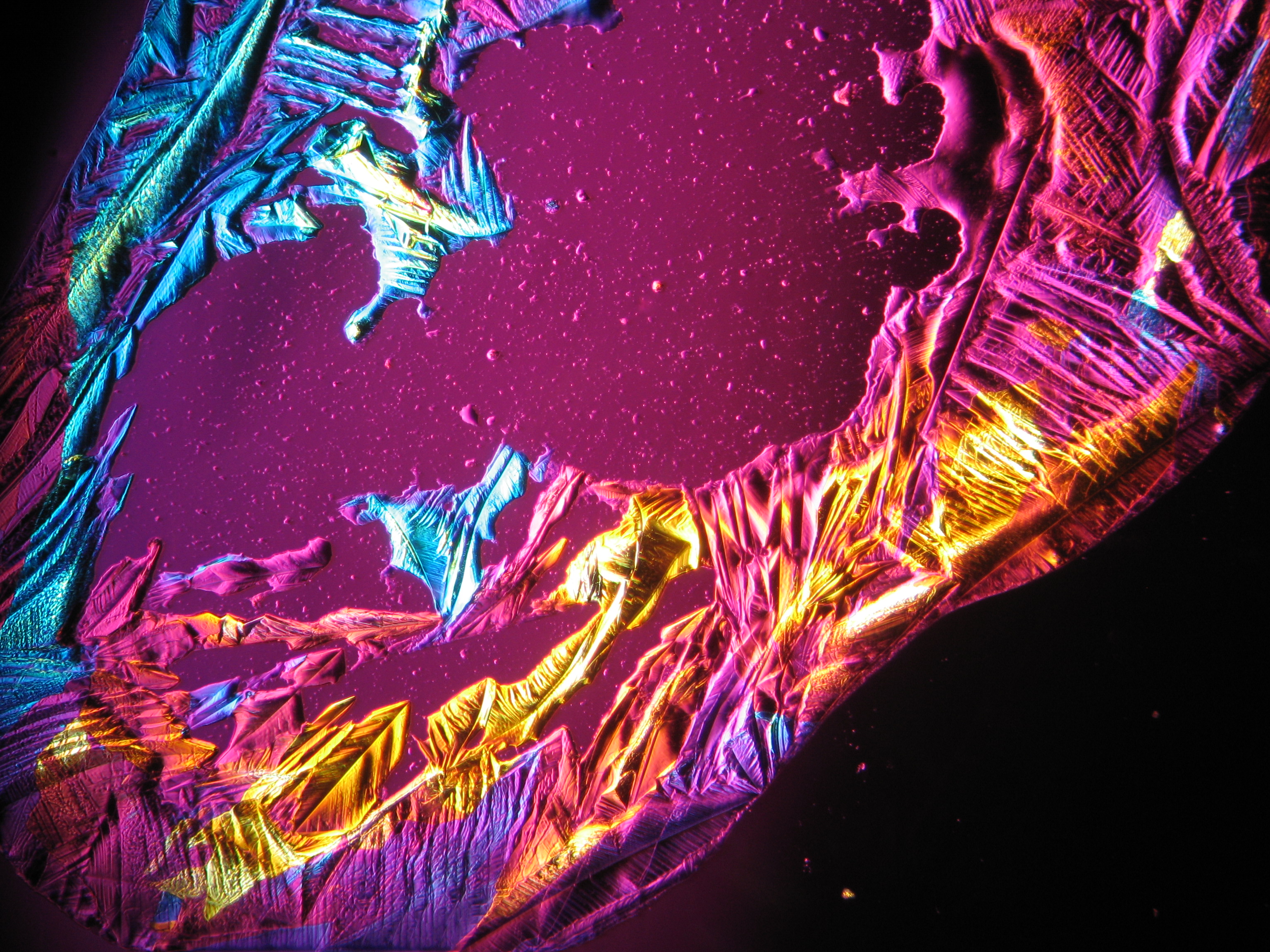
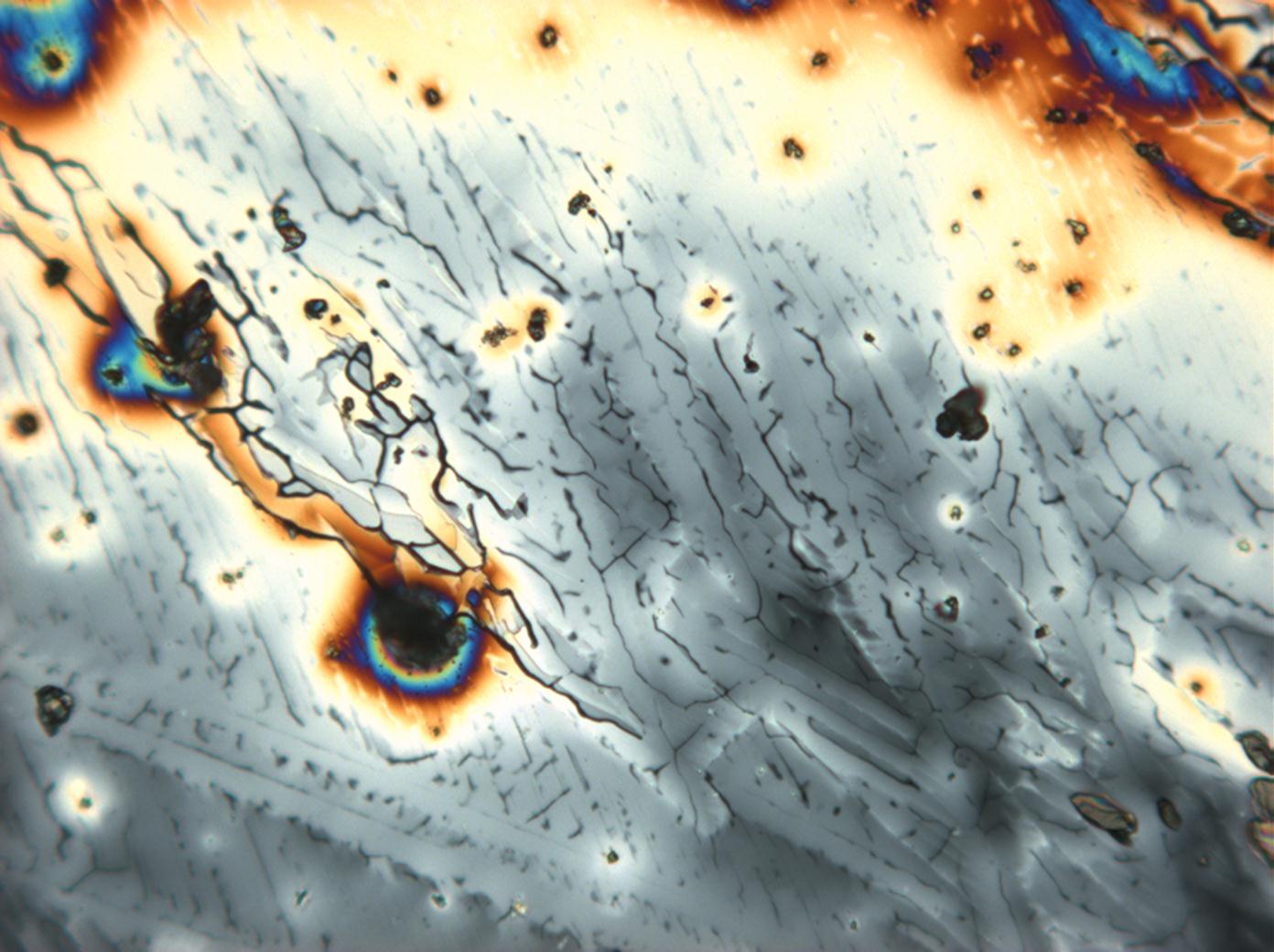
Under the polarising microscope[edit]
- Crystallized from a water solution on a glass slide
Weblinks[edit]
- ↑ http://webmineral.com/data/Epsomite.shtml seen on 29.072010
- ↑ http://www.mindat.org/min-1393.html seen on 29.072010