Magnesium sulfate: Difference between revisions
No edit summary |
No edit summary |
||
| (One intermediate revision by one other user not shown) | |||
| Line 50: | Line 50: | ||
<br clear="all"> | <br clear="all"> | ||
[[File:S MgSO4.jpg|thumb|800px| | [[File:S MgSO4.jpg|thumb|800px|left|'''Figure 1:''' Solubilities in the system MgSO<sub>4</sub>-H<sub>2</sub>O and its temperature dependence. The molality ''m'' [n(MgSO<sub>4</sub>•xH<sub>2</sub>O)•kg(H<sub>2</sub>O)<sup>-1</sup>] is plotted versus the temperature. Equilibria of the different hydrated forms can be distiguished by the different colours of the curves. Dotted lines represent the metastable equilibria. In addition to the solubilities, freezing and boiling points are given. According to <bib id="Steiger.etal:2011a"/>.]] | ||
<br clear="all"> | <br clear="all"> | ||
| Line 76: | Line 76: | ||
|bgcolor = "#FFFFEO" align=center| 39.1 % | |bgcolor = "#FFFFEO" align=center| 39.1 % | ||
|} | |} | ||
<br clear="all"> | |||
accompanied by a hydration or a dehydration of a considered phase. | |||
<br clear="all"> | |||
[[File:D MgSO4 e.jpg|thumb|800px|left|'''Figure 2:''' Deliquescence behaviour in the system MgSO<sub>4</sub>-H<sub>2</sub>O. The water activity ''a<sub>w</sub>'' is plotted versus the temperature. Different colours label equilibrium and deliquescence humidities of the different phases of the system. Dotted lines represent metastable equilibria. According to <bib id="Steiger.etal:2011a"/>.]] | |||
<br clear="all"> | <br clear="all"> | ||
Latest revision as of 11:05, 3 May 2023
Author: Amelie Stahlbuhk
back to Sulfate
| This article will be released soon. |
Abstract[edit]
The different hydrated forms of magnesium sulfate and the behaviour concerning solubility and hygroscopicity will be presented.
Hydrated forms[edit]
Kieserite MgSO4•H2O
Sanderite MgSO4•2H2O
Starkeyite MgSO4•4H2O
Pentahydrite MgSO4•5H2O
Hexahydrite MgSO4•6H2O
Epsomite MgSO4•7H2O
Meridianiite MgSO4•11H2O
Solubility[edit]
As it is shown in table 1, the different hydrated forms of magnesium sulfate are easily soluble salts, which leads to a high mobility of the salts in porous materials.
| Hydrated form | Solubility [mol/kg] at 20°C |
| Kieserite | 5.60 |
| Starkeyite | 5.04 |
| Pentahydrite | 4.40 |
| Hexahydrite | 3.61 |
| Epsomite | 2.84 |
Due to the different hydrated forms of magnesium sulfate with stable and meta stable equilibria, the solubility diagram of the system MgSO4-H2O contains more information than diagrams of salts with less or also without any hydrated forms. With the temperature dependence of the solubility it is possible that temperature changes are accompanied by a hydration or a dehydration of a considered phase.
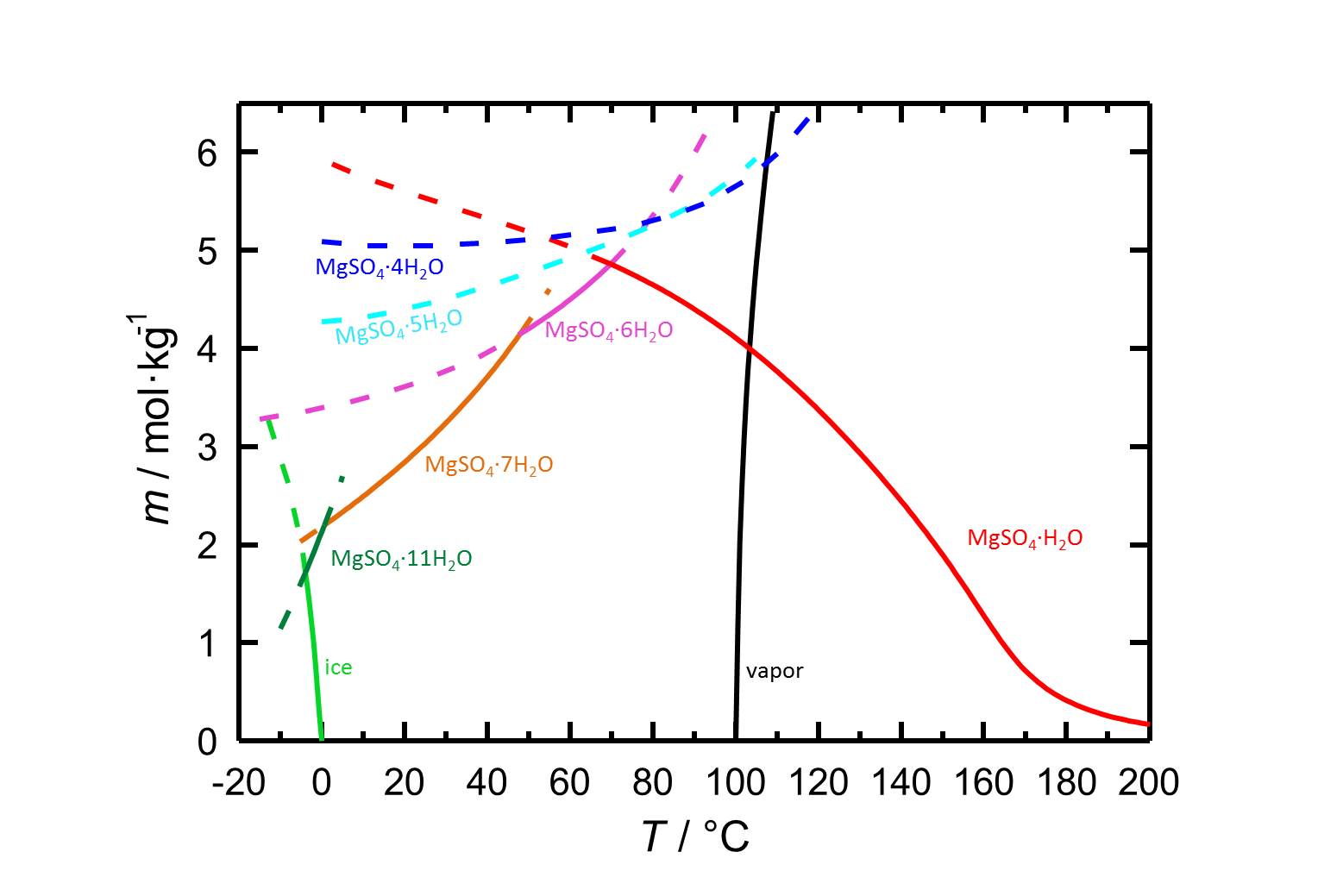
Author: Steiger, Michael; Linnow, Kirsten; Ehrhardt, Dorothee; Rohde, Mandy
 .
.
Hygroscopicity[edit]
In the system MgSO4-H2O changes in temperature or relative humidity may lead to hydration/dehydration or deliquescence/crystallization processes. At 20 °C epsomite is the present crystalline phase when the relative humidity is below its deliquescence humidity of 91.3 %. When the relative humidity reaches values below 47 % the dehydration to lower hydrated levels sets in, as it is represented by the curves of the equilibrium humidities in figure 2.
| Phase transition | Deliquescence or equilibrium humidity at 20°C |
| Epsomite-solution | 91.3 % |
| Epsomite-Hexahydrite | 46.6 % |
| Epsomite-Kieserite | 46.7 % |
| Hexahydrite-Starkeyite | 39.1 % |
accompanied by a hydration or a dehydration of a considered phase.
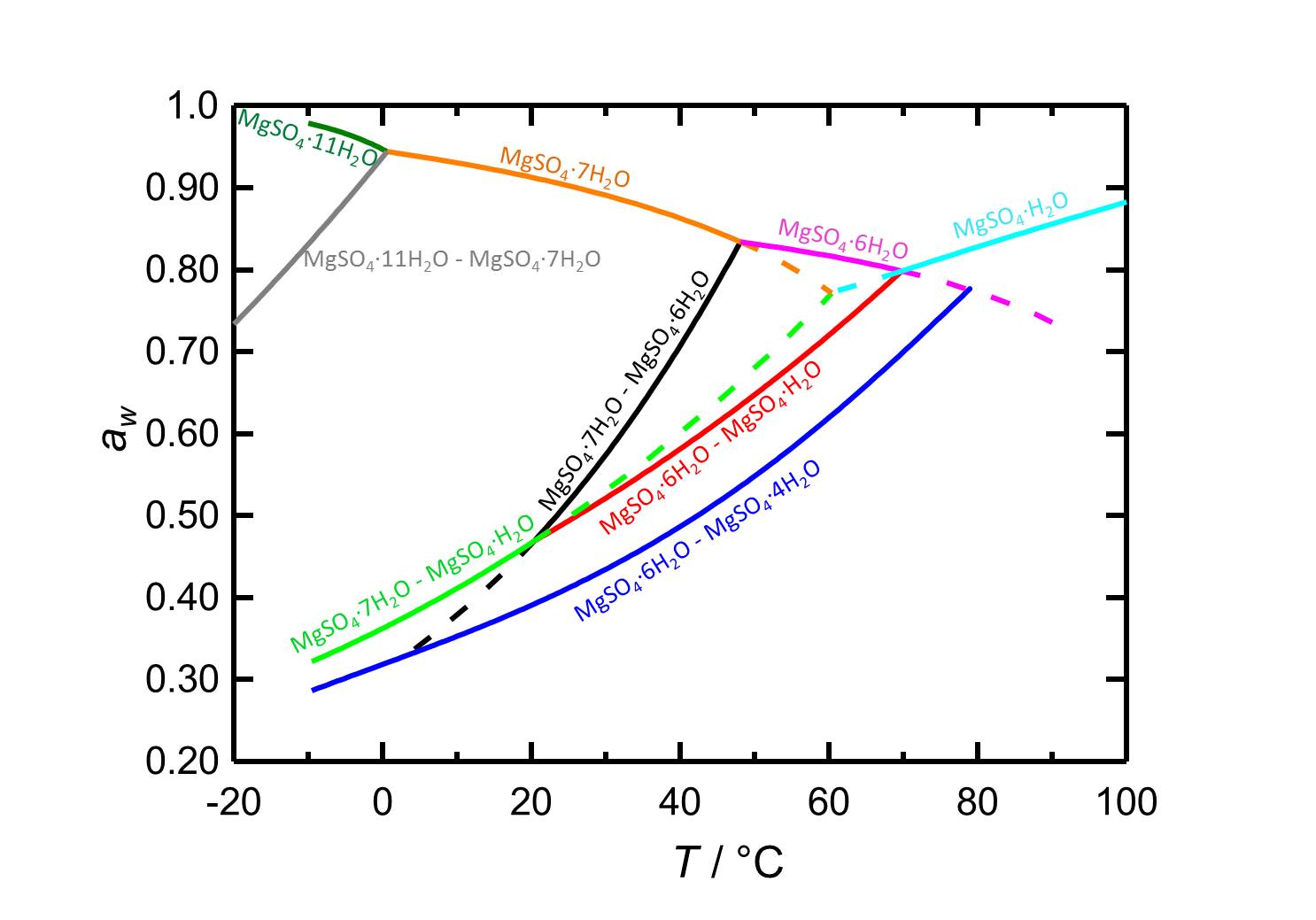
Author: Steiger, Michael; Linnow, Kirsten; Ehrhardt, Dorothee; Rohde, Mandy
 .
.
In the temperature range of -10 to 100 °C the deliquescence humidities of the present hydrated forms (depending on the temperature) lie always above 80 % r.h., so the salts do not belong to the hygroscopic salts.
Weblinks[edit]
Literature[edit]
| [Mainusch:2001] | Mainusch, Nils (2001): Erstellung einer Materialsammlung zur qualitativen Bestimmung bauschädlicher Salze für Fachleute der Restaurierung, Diplomarbeit, HAWK Hochschule für angewandte Wissenschaft und Kunst Hildesheim/Holzminden/Göttingen, file:Diplomarbeit Nils Mainusch.pdf |   |
| [Stark.etal:1996] | Stark, Jochen; Stürmer, Sylvia (1996): Bauschädliche Salze, Bauhaus-Univ. Weimar |  |
| [Steiger.etal:2011a] | Steiger, Michael; Linnow, Kirsten; Ehrhardt, Dorothee; Rohde, Mandy (2011): Decomposition reactions of magnesium sulfate hydrates and phase equilibria in the MgSO4-H2O and Na+-Mg2+-Cl--SO42--H2O systems with implications for Mars. In: Geochimica et Cosmochimica Act, 75 (12), 3600-3626, 10.1016/j.gca.2011.03.038, |  |