Niter: Difference between revisions
No edit summary |
No edit summary |
||
| (8 intermediate revisions by one other user not shown) | |||
| Line 34: | Line 34: | ||
== Abstract == | == Abstract == | ||
Niter is one of the most important salts responsible for damages of building materials and murals. It is mostly observed indoors, often found in cotton-like efflorescence. Pictures, microphotographs and examples of habits illustrate and complement the description. | Niter is one of the most important salts responsible for damages of building materials and murals. It is mostly observed indoors, often found in cotton-like efflorescence. Pictures, microphotographs and examples of their habits illustrate and complement the description. | ||
== General == | == General == | ||
| Line 43: | Line 43: | ||
== Occurrence of Niter == | == Occurrence of Niter == | ||
Natural accumulations of potassium nitrate can occur anywhere where nitrogen compounds are synthesized in presence of a sufficient quantity of potassium ions (for example during the decomposition of organic matter). Large quantities of nitrifying bacteria and nitrogen compounds are known to be present in manure and urine of living organisms. Large deposits of potassium and sodium nitrate, called "salitre" in Spanish, are found in the desert region of Atacama, nothern Chile, which have been exploited since the 19th century. | |||
== Information on the origin and formation of Niter on monuments == | == Information on the origin and formation of Niter on monuments == | ||
Contaminated | Contaminated water at the base of monuments is the major input source of both potassium and nitrate ions, the latter having a biogenic origin, such as nitrifying bacteria. These ions will be transported into the material via capillarity. Nitrate ions can also be deposited from atmospheric pollution. Once within the porous system of the material, creep of the formed salt will also contribute to its redistribution. In addition, building and restoration materials can also contain soluble potassium compounds. We can also mention potassium silicate, potassium hydroxide (used as a cleaning agent)and cements.<br> | ||
== Solution behavior== | == Solution behavior== | ||
| Line 64: | Line 63: | ||
== Hygroscopicity== | == Hygroscopicity== | ||
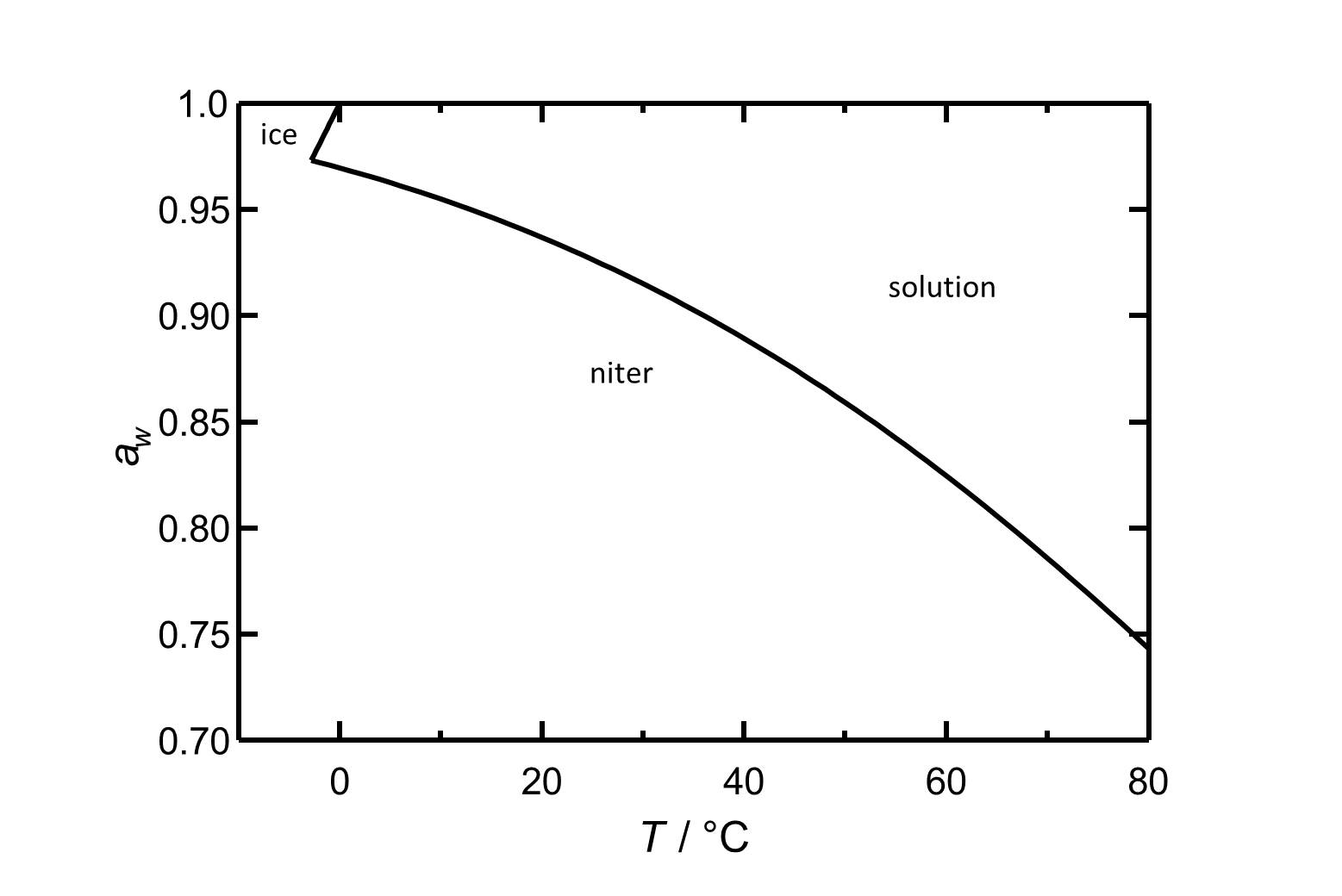
[[file:D KNO3 e.jpg|thumb|800px| | In the temperature range of 0°C up to 30°C the [[deliquescence humidity]] of potassium nitrate lies always above 90% RH and the temperature dependence is more or less linear. Under the influence of foreign ions the deliquescence humidity is shifted towards lower values. | ||
[[file:D KNO3 e.jpg|thumb|800px|left|'''Figure 2''':Deliquescence behaviour of potassium nitrate. The water activity ''a<sub>w</sub>'' is plotted versus the temperature.]] | |||
<br clear="all"> | <br clear="all"> | ||
| Line 80: | Line 81: | ||
|- | |- | ||
|bgcolor = "#FFFFEO" align=center| 97.0% | |bgcolor = "#FFFFEO" align=center| 97.0% RH | ||
|bgcolor = "#FFFFEO" align=center| 95.5% | |bgcolor = "#FFFFEO" align=center| 95.5% RH | ||
|bgcolor = "#FFFFEO" align=center| 93.7% | |bgcolor = "#FFFFEO" align=center| 93.7% RH | ||
|bgcolor = "#FFFFEO" align=center| 91.5% | |bgcolor = "#FFFFEO" align=center| 91.5% RH | ||
|bgcolor = "#FFFFEO" align=center| 88.9% | |bgcolor = "#FFFFEO" align=center| 88.9% RH | ||
|bgcolor = "#FFFFEO" align=center| 85.9% | |bgcolor = "#FFFFEO" align=center| 85.9% RH | ||
|} | |} | ||
| Line 194: | Line 195: | ||
= Dealing with damage caused by Niter = | = Dealing with damage caused by Niter = | ||
--> | --> | ||
== Niter and damages caused by niter in the image == | == Niter and damages caused by niter in the image == | ||
| Line 201: | Line 201: | ||
<gallery caption=" " widths="200px" heights="150px" perrow="3"> | <gallery caption=" " widths="200px" heights="150px" perrow="3"> | ||
Image:KNO3-SalzflaumKoenigslutter.jpg| fluffy salt | Image:KNO3-SalzflaumKoenigslutter.jpg| fluffy salt crystals of niter break-up the surface | ||
Image: | Image: | ||
Image: | Image: | ||
| Line 211: | Line 211: | ||
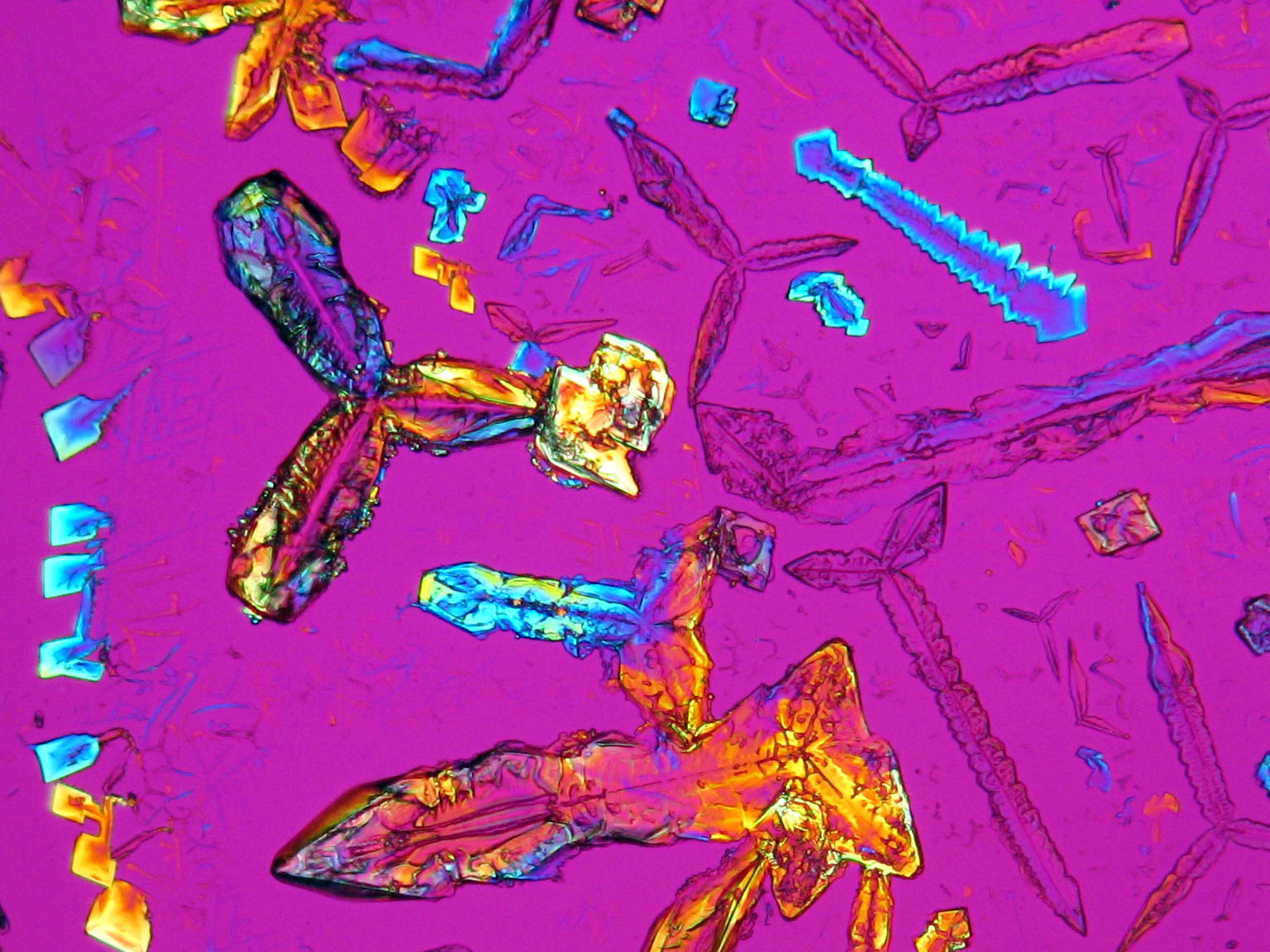
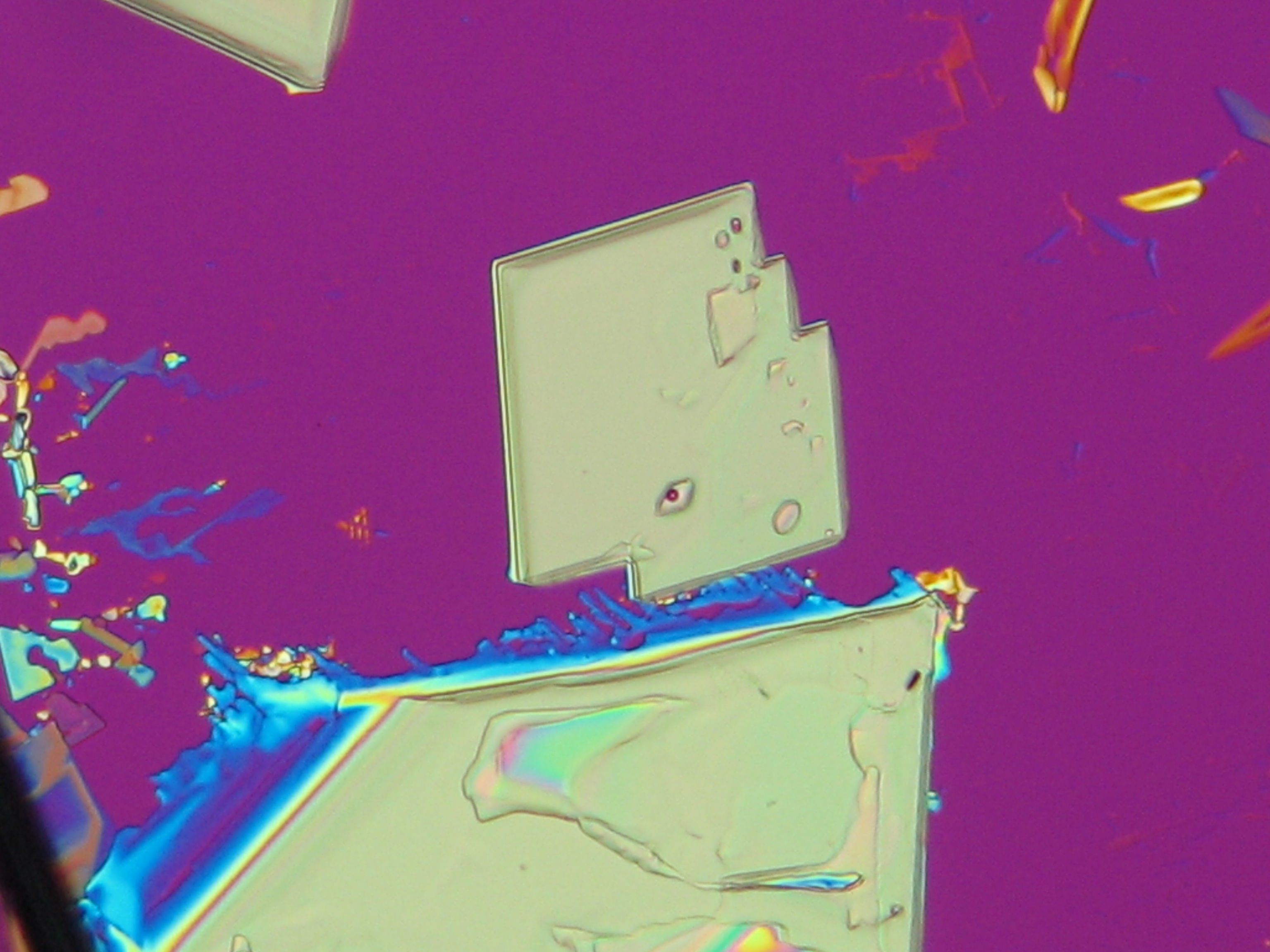
=== Under the polarizing microscope === | === Under the polarizing microscope === | ||
<gallery caption=" | <gallery caption="KNO3 crystallized from aqueous solution on a slide" widths="200px" heights="150px" perrow="3"> | ||
Image:HJS KNO3 092503-6.jpg | in simple polarized light | Image:HJS KNO3 092503-6.jpg | in simple polarized light | ||
Image:HJS KNO3 092503-5.jpg| under crossed polarizers | Image:HJS KNO3 092503-5.jpg| under crossed polarizers | ||
| Line 253: | Line 253: | ||
[[Category:Schwarz,Hans-Jürgen]] | [[Category:Schwarz,Hans-Jürgen]] | ||
[[Category:R-MSteiger]] | [[Category:R-MSteiger]] | ||
[[Category: | [[Category:complete]] | ||
[[Category:Nitrate]] | [[Category:Nitrate]] | ||
[[Category:Salt]] | [[Category:Salt]] | ||
[[Category:List]] | [[Category:List]] | ||
Latest revision as of 11:47, 3 May 2023
Authors: Hans-Jürgen Schwarz, Nils Mainusch
Translation of the German Version by Hans-Jürgen Schwarz
back to Nitrate
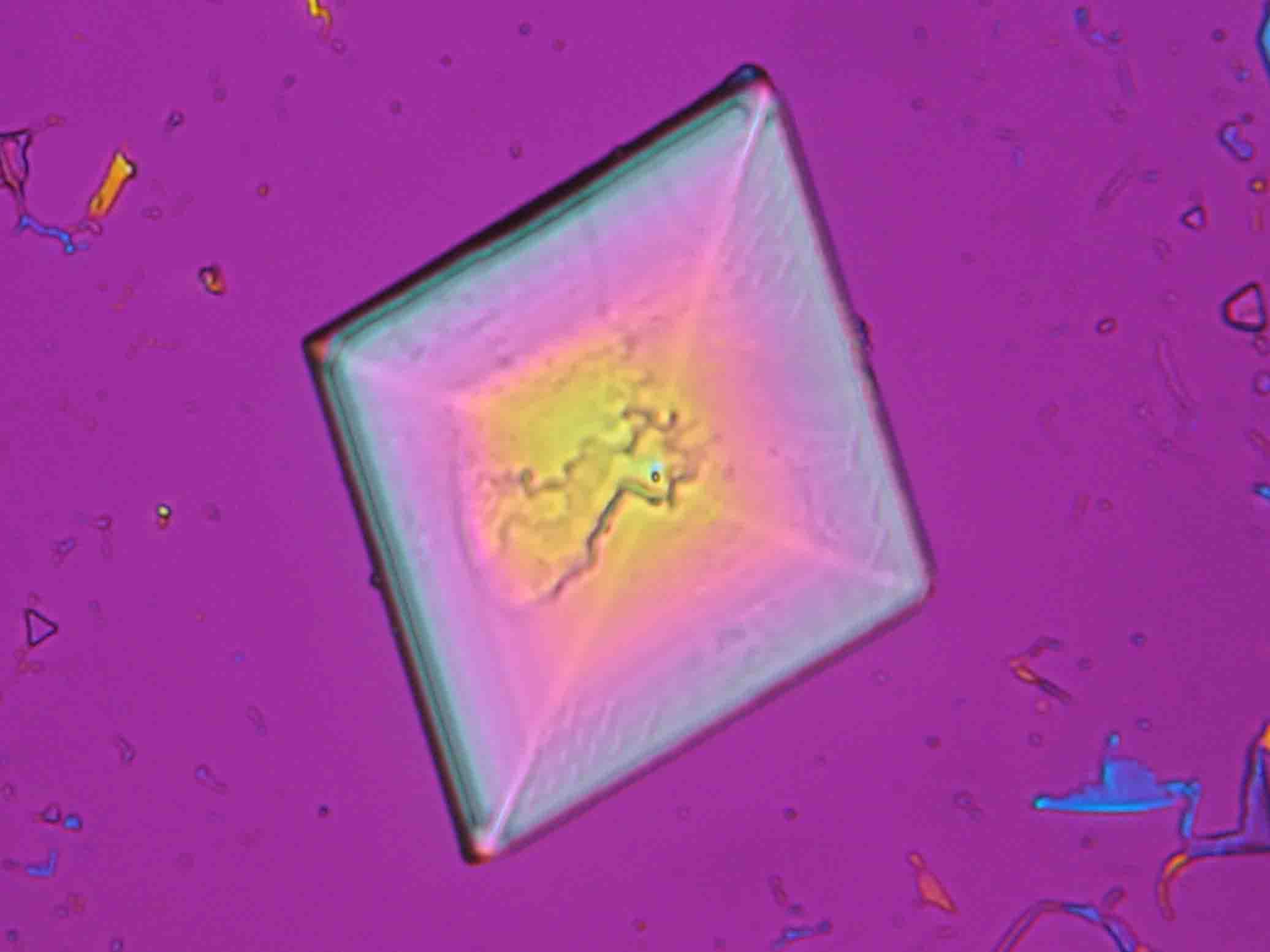
| Niter[1][2][3] | |
| |
| Mineralogical name | Nitrokalite, Niter, Kalisalpeter |
| Chemical name | Potassium nitrate |
| Trivial name | Saltpetre , Nitrate of potash, Vesta powder, Kali-Salpeter, Kehrsalpeter, Konversionssalpeter |
| Chemical formula | KNO3 |
| Other forms | none |
| Crystal system | orthorhombic |
| Crystal structure | orthorhombic - dipyramidal; 2/m 2/m 2/m , see [4] |
| Deliquescence humidity 20°C | 93.7 % |
| Solubility (g/l) at 20°C | 3.108 mol/kg |
| Density (g/cm³) | 2.103 g/cm3 |
| Molar volume | 48.04 cm3/mol |
| Molar weight | 101.10 g/mol |
| Transparency | translucent to transparent |
| Cleavage | very good on {001}; good on {010}h[5] |
| Crystal habit | include crusts and acicular crystals formed as efflorescence on cave and mine walls [6] |
| Twinning | |
| Phase transition | |
| Chemical behavior | easily soluble in water |
| Comments | |
| Crystal Optics | |
| Refractive Indices | α = 1.335 β = 1.505 γ = 1.506 |
| Birefringence | Δ = 0.171 |
| Optical Orientation | biaxial negative |
| Pleochroism | |
| Dispersion | weak, r < v |
| Used Literature | |
| [Steiger.etal:2014]Title: Weathering and Deterioration Author: Steiger, Michael; Charola A. Elena; Sterflinger, Katja  [Robie.etal:1978]Title: Thermodynamic properties of minerals and related substances at 298.15 K and 1 bar pressure and higher temperatures [Robie.etal:1978]Title: Thermodynamic properties of minerals and related substances at 298.15 K and 1 bar pressure and higher temperaturesAuthor: Robie R.A., Hemingway B.S.; Fisher J.A. 
| |
Abstract[edit]
Niter is one of the most important salts responsible for damages of building materials and murals. It is mostly observed indoors, often found in cotton-like efflorescence. Pictures, microphotographs and examples of their habits illustrate and complement the description.
General[edit]
Potassium nitrate was an important ingredient in the production of gun powder and explosives in the 19th century, were obtained from natural sources potash deposits contain a maximum of 10% KNO3 among various other potassium salts) and obtained through the reaction of sodium nitrate with potassium chloride. Currently various methods of large-scale production of this salt are based on the reaction between crude potassium salts and nitric acid.
Potassium nitrate is also used as a fertilizer in agriculture.
Occurrence of Niter[edit]
Natural accumulations of potassium nitrate can occur anywhere where nitrogen compounds are synthesized in presence of a sufficient quantity of potassium ions (for example during the decomposition of organic matter). Large quantities of nitrifying bacteria and nitrogen compounds are known to be present in manure and urine of living organisms. Large deposits of potassium and sodium nitrate, called "salitre" in Spanish, are found in the desert region of Atacama, nothern Chile, which have been exploited since the 19th century.
Information on the origin and formation of Niter on monuments[edit]
Contaminated water at the base of monuments is the major input source of both potassium and nitrate ions, the latter having a biogenic origin, such as nitrifying bacteria. These ions will be transported into the material via capillarity. Nitrate ions can also be deposited from atmospheric pollution. Once within the porous system of the material, creep of the formed salt will also contribute to its redistribution. In addition, building and restoration materials can also contain soluble potassium compounds. We can also mention potassium silicate, potassium hydroxide (used as a cleaning agent)and cements.
Solution behavior[edit]
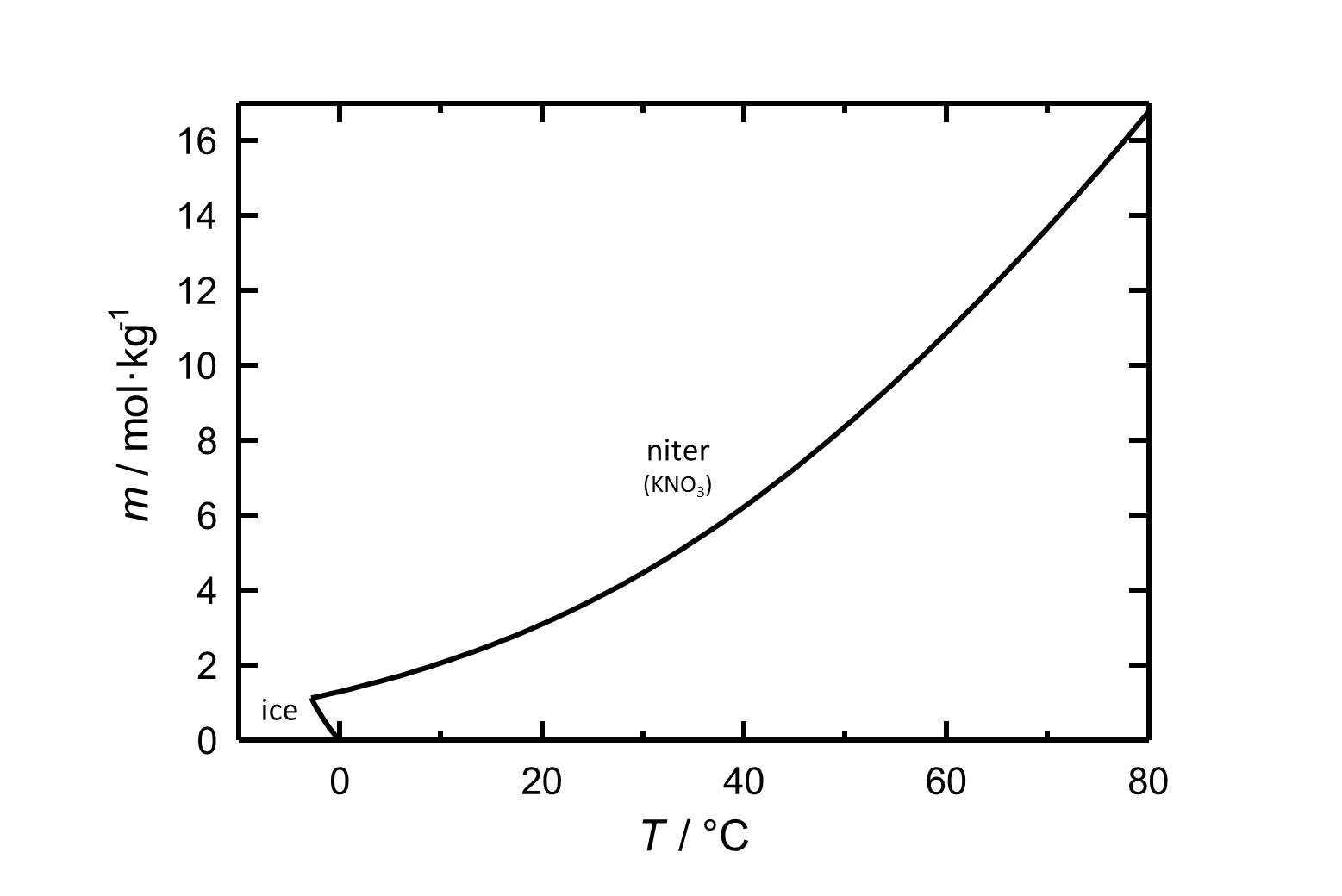
Potassium nitrate has a high water solubility, belonging to the group of mobile salts. It can be associated with frequent changes in the accumulation zones in the porous material. The temperature effect on the water solubility is strong, which is reflected in the steep curve in figure 1.
A consequence of this is the danger of solution supersaturation during rapid temperature drop, and a subsequent crystallization of the salt.
Hygroscopicity[edit]
In the temperature range of 0°C up to 30°C the deliquescence humidity of potassium nitrate lies always above 90% RH and the temperature dependence is more or less linear. Under the influence of foreign ions the deliquescence humidity is shifted towards lower values.
| 0°C | 5°C | 10°C | 20°C | 30°C | 40°C |
| 97.0% RH | 95.5% RH | 93.7% RH | 91.5% RH | 88.9% RH | 85.9% RH |
Niter and damages caused by niter in the image[edit]
On objects[edit]
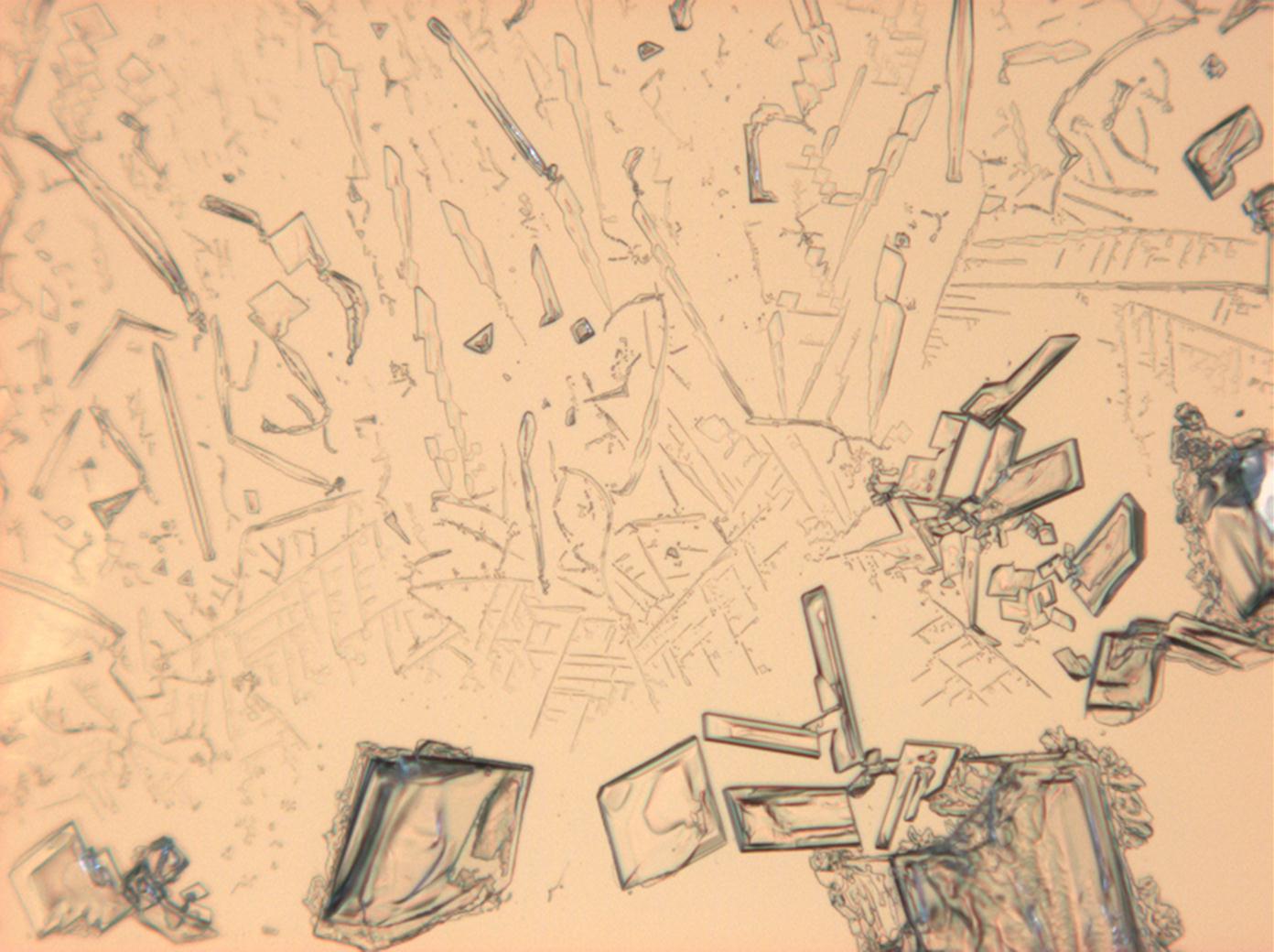
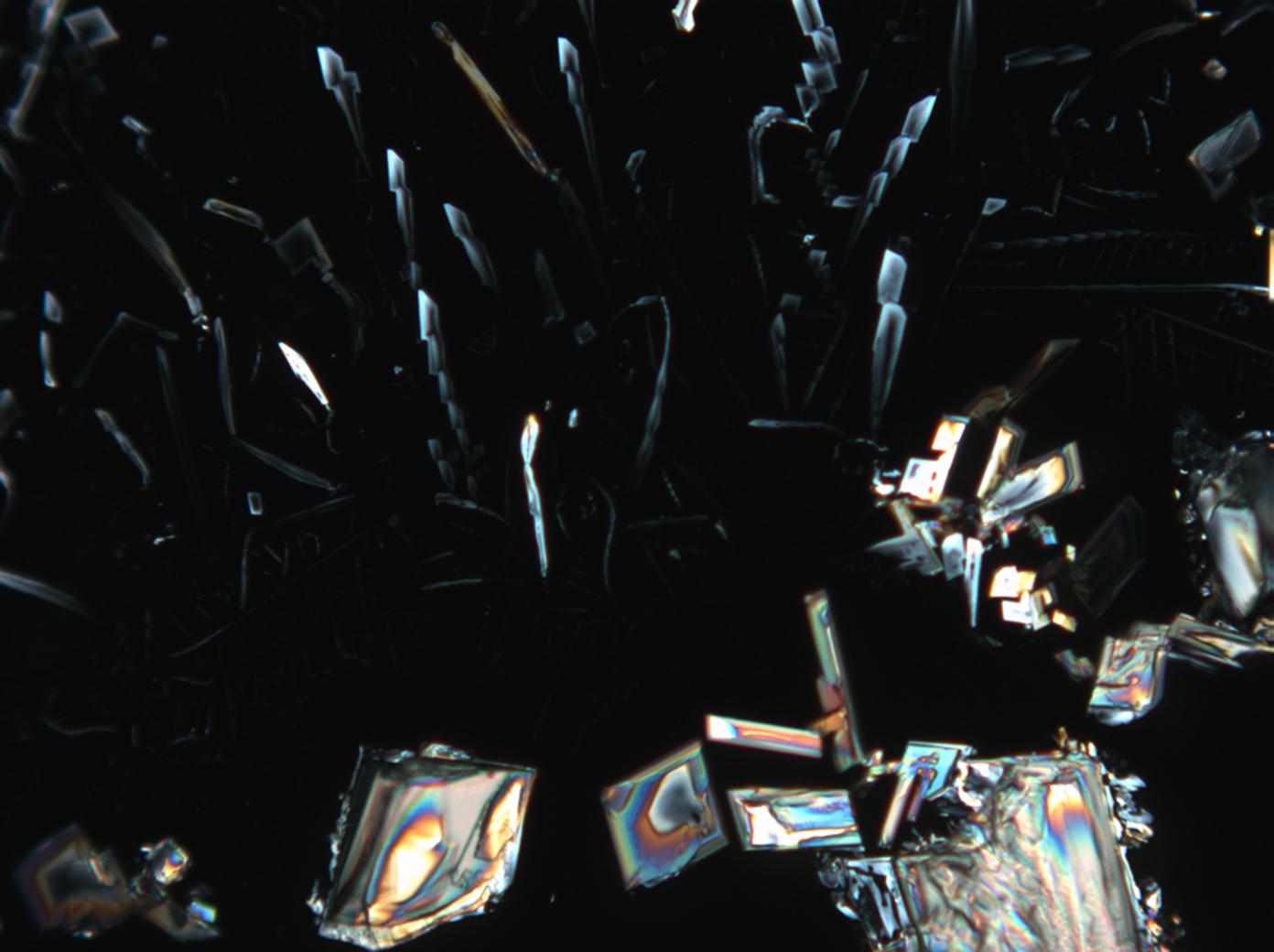
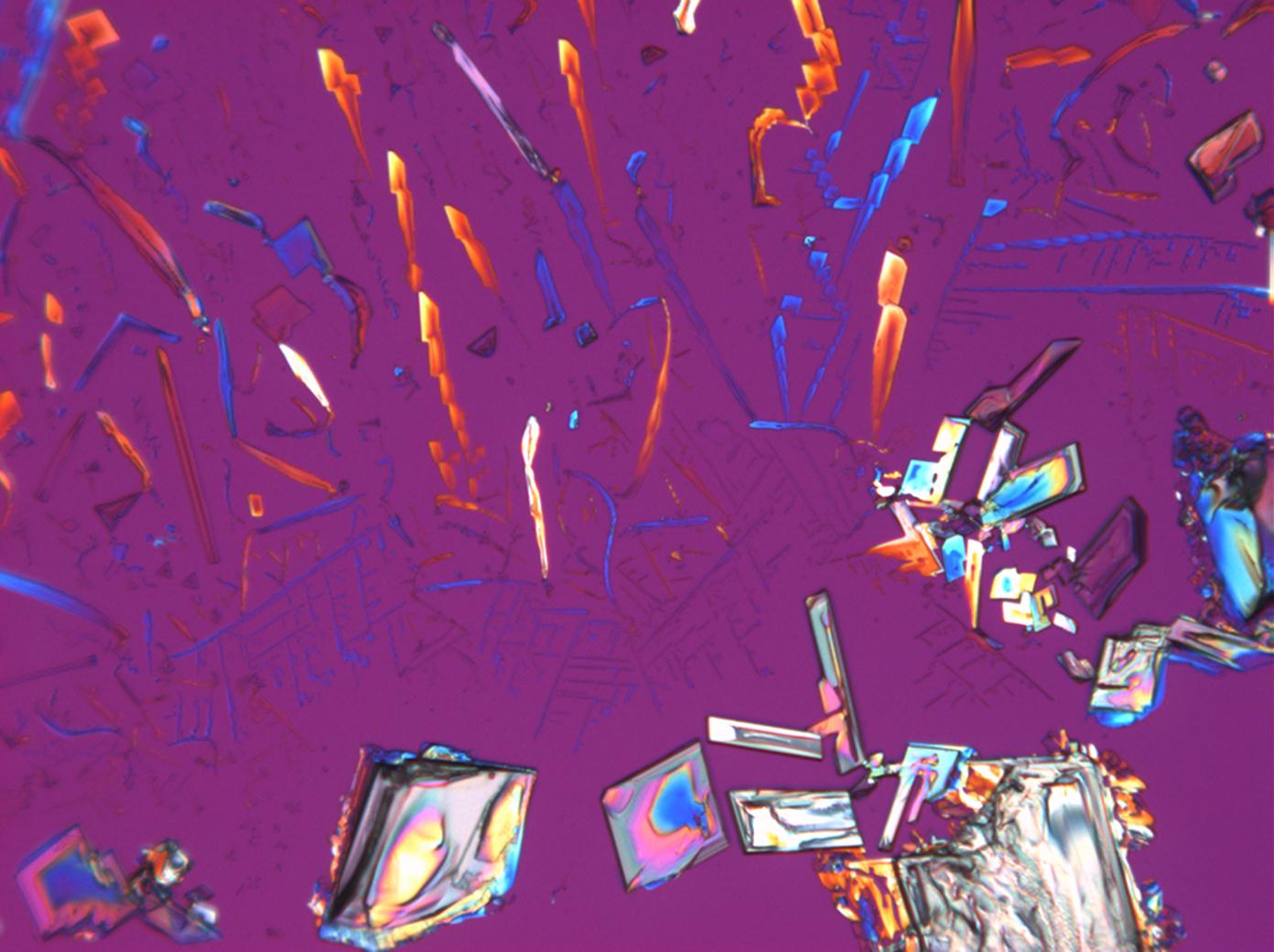
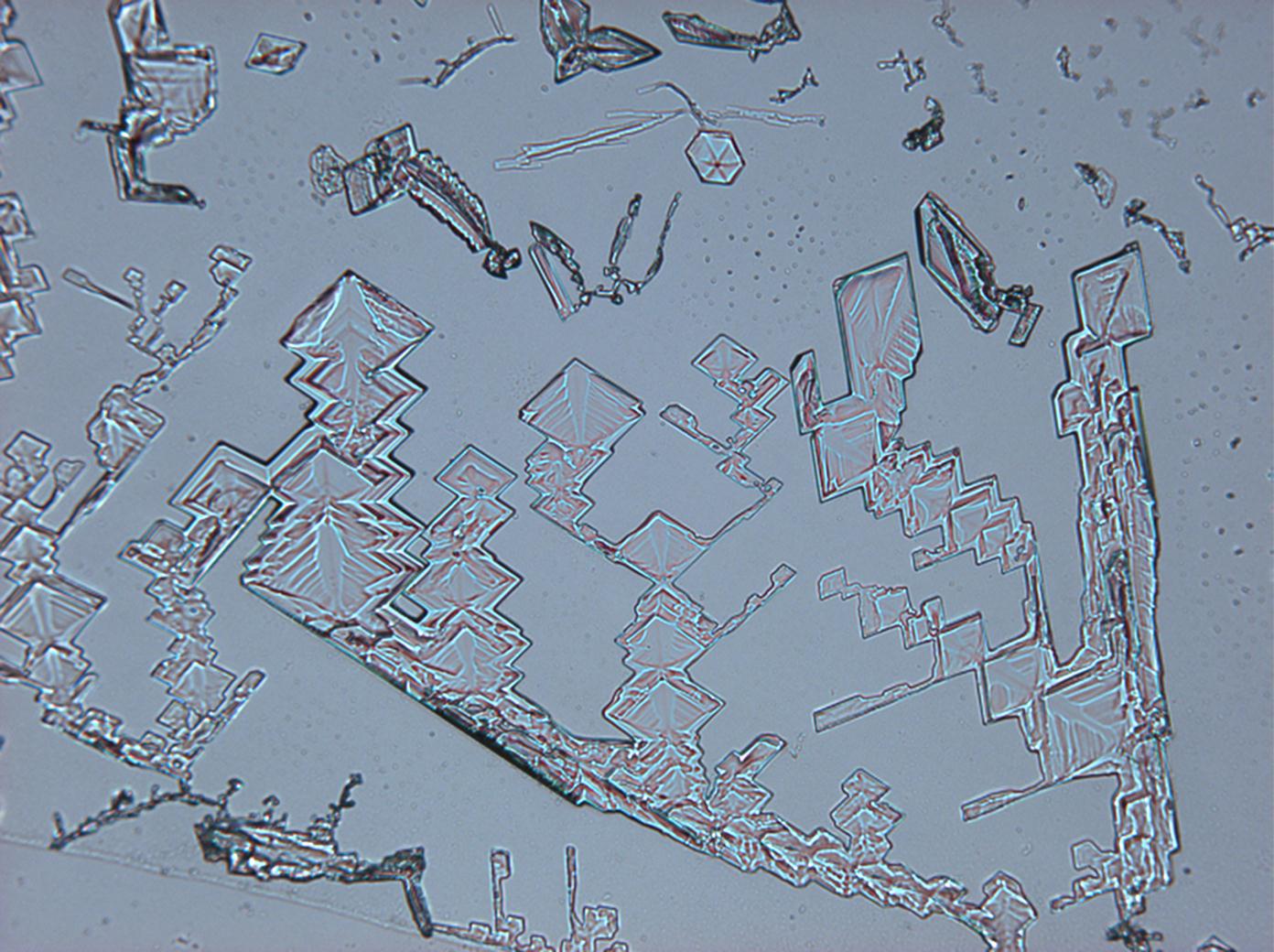
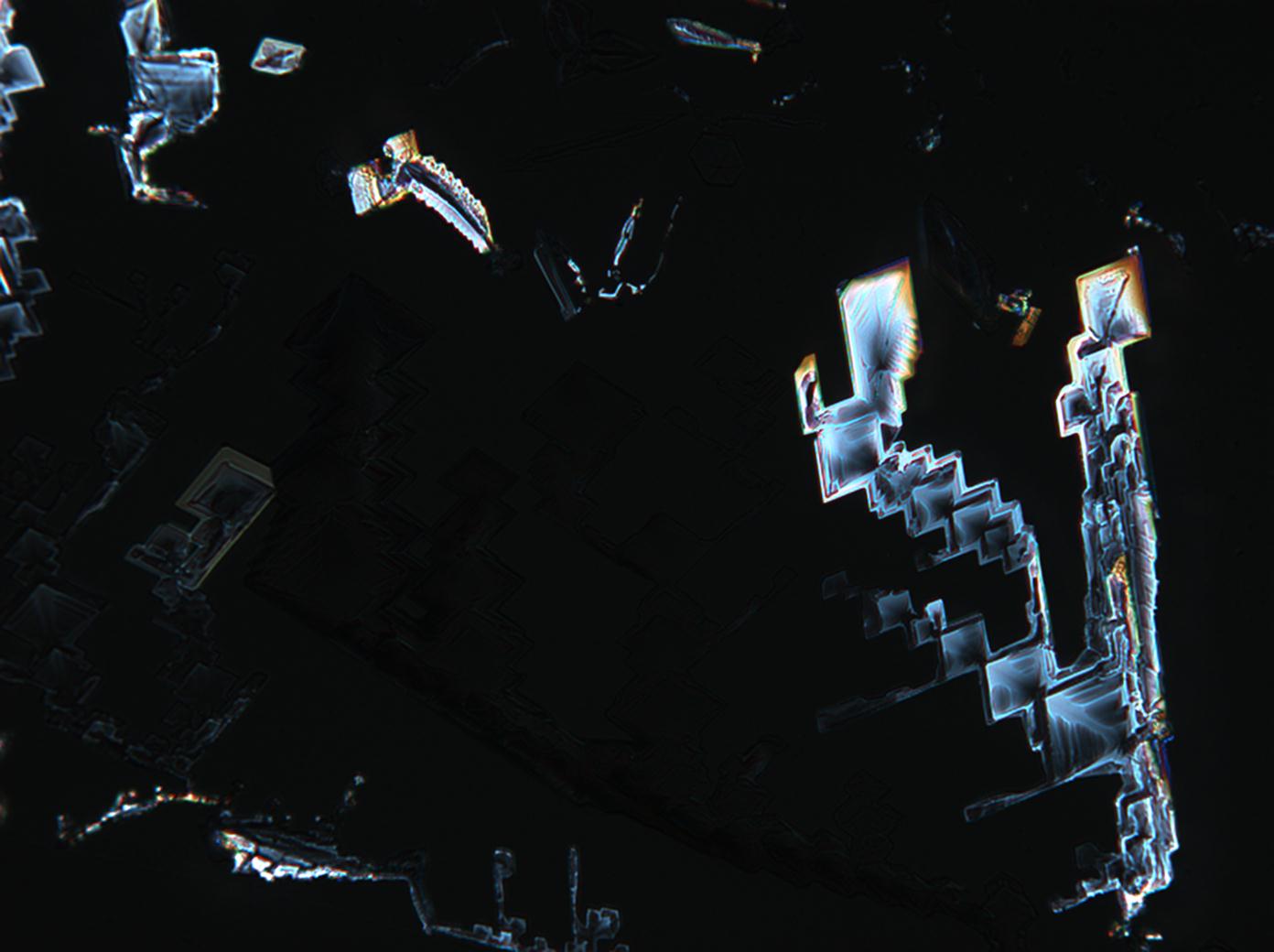
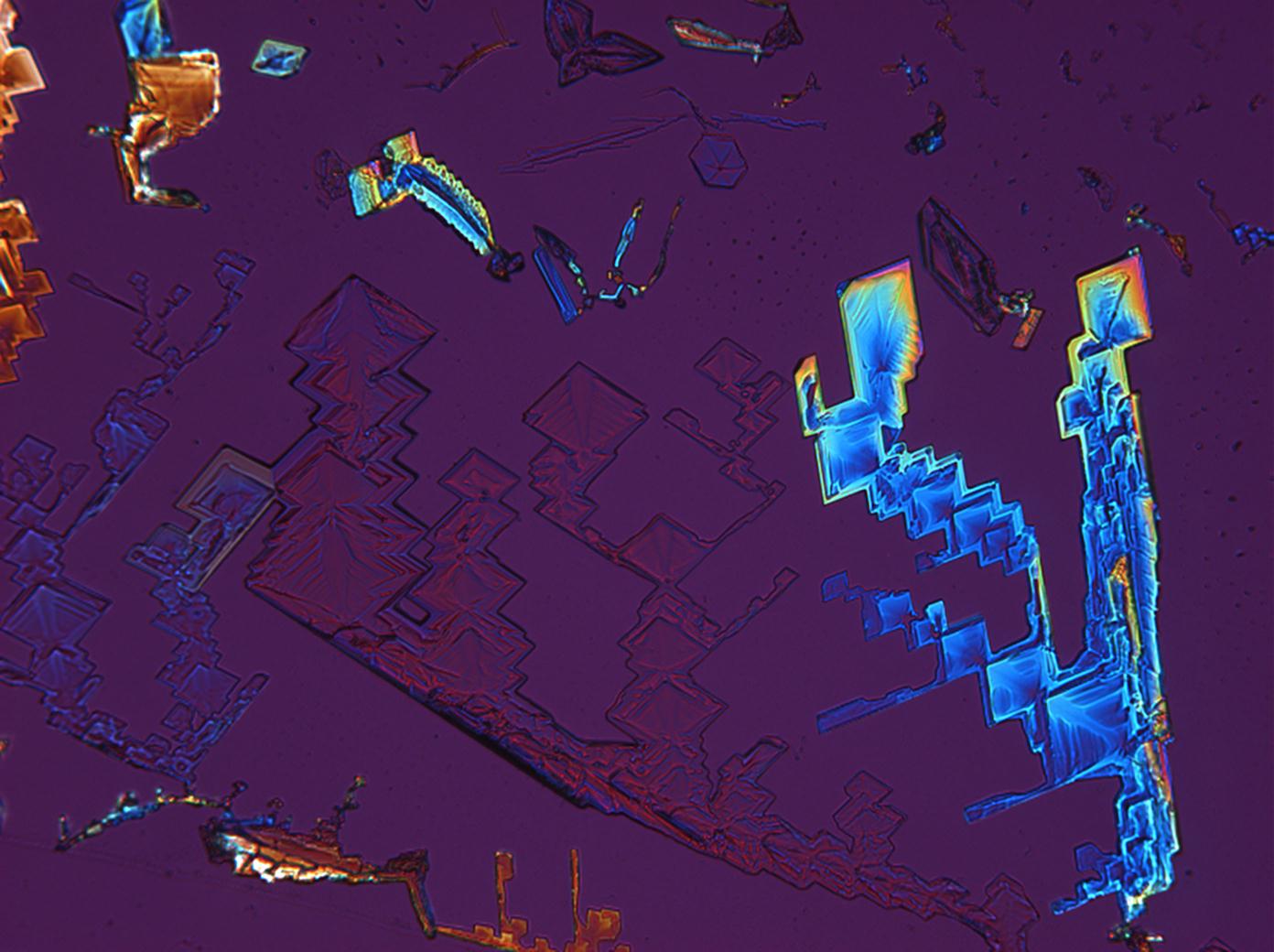
Under the polarizing microscope[edit]
- KNO3 crystallized from aqueous solution on a slide
Under the scanning electron microscope[edit]
Weblinks[edit]
- ↑ http://webmineral.com/data/Niter.shtml gelesen 28.07.2010
- ↑ http://www.mindat.org/min-2917.html
- ↑ http://www.mineralienatlas.de/lexikon/index.php/MineralData?mineral=Niter
- ↑ http://webmineral.com/jpowd/JPX/jpowd.php?target_file=Niter.jpx; Viewed on 15/04/2011
- ↑ ttp://en.wikipedia.org/wiki/Niter; Viewed on 15/04/2011
- ↑ http://www.galleries.com/minerals/carbonat/niter/niter.htm; Viewed on 15/04/2011
Literature[edit]
| [Mainusch:2001] | Mainusch, Nils (2001): Erstellung einer Materialsammlung zur qualitativen Bestimmung bauschädlicher Salze für Fachleute der Restaurierung, Diplomarbeit, HAWK Hochschule für angewandte Wissenschaft und Kunst Hildesheim/Holzminden/Göttingen, file:Diplomarbeit Nils Mainusch.pdf |   |
| [Robie.etal:1978] | Robie R.A., Hemingway B.S.; Fisher J.A. (1978): Thermodynamic properties of minerals and related substances at 298.15 K and 1 bar pressure and higher temperatures. In: U.S. Geol. Surv. Bull, 1452 () |  |
| [Steiger.etal:2014] | Steiger, Michael; Charola A. Elena; Sterflinger, Katja (2014): Weathering and Deterioration. In: Siegesmund S.; Snethlage R. (eds.): Stone in Architecture, Springer Verlag Berlin Heidelberg, 223-316, 10.1007/978-3-642-45155-3_4. |  |
| [Vogt.etal:1993] | Vogt, R.; Goretzki, Lothar (1993): Der Einfluss hygroskopischer Salze auf die Gleichgewichtsfeuchte und Trocknung anorganischer Baustoffe, unveröffentlichter Bericht. |  |