Nitronatrite
Revision as of 15:14, 28 January 2015 by AmelieStahlbuhk (talk | contribs)
| Nitronatrite[1][2] | |
| |
| Mineralogical name | Sodium Nitrate |
| Chemical name | Natriumnitrat |
| Trivial name | Nitratine, Nitratite, Soda Niter, Cubic Niter |
| Chemical formula | NaNO3 |
| Other forms | |
| Crystal system | trigonal |
| Crystal structure | |
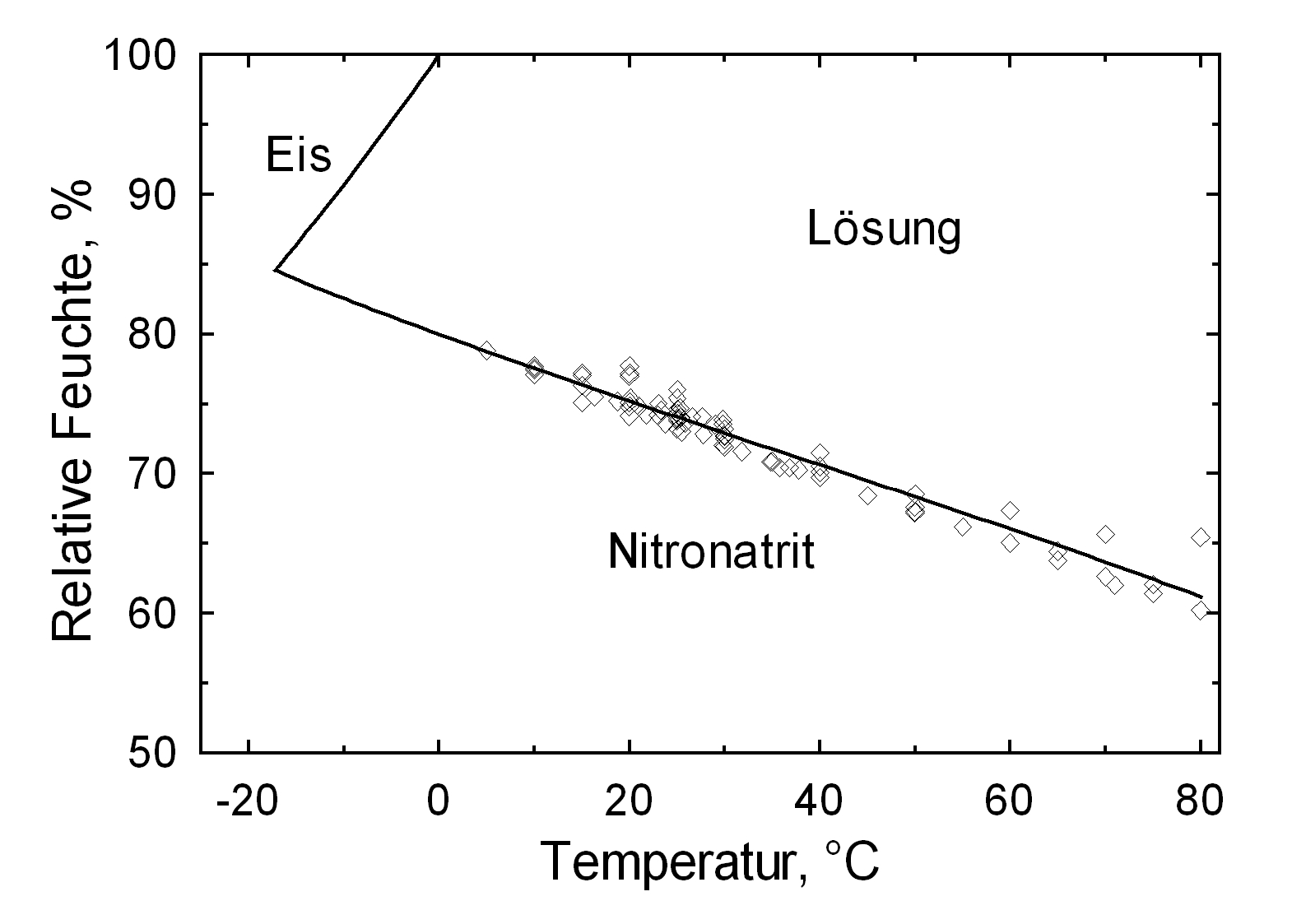
| Deliquescence humidity 20°C | 75.3% [Steiger etal: 2014]Title: Weathering and Deterioration Author: Steiger, Michael; Charola A. Elena; Sterflinger, Katja 
|
| Solubility (g/l) at 20°C | 880 g/l |
| Density (g/cm³) | 2.24-2.29 g/cm3 |
| Molar volume | 37.6 cm3/mol |
| Molar weight | 85 g/mol |
| Transparency | transparent |
| Cleavage | perfect |
| Crystal habit | |
| Twinning | |
| Phase transition | |
| Chemical behavior | |
| Comments | |
| Crystal Optics | |
| Refractive Indices | no = 1.587 ne = 1.336 |
| Birefringence | Δ = 0.251 |
| Optical Orientation | |
| Pleochroism | |
| Dispersion | |
| Used Literature | |
| {{{Literature}}} | |
Authtor: Hans-Jürgen schwarz
back to Nitrate
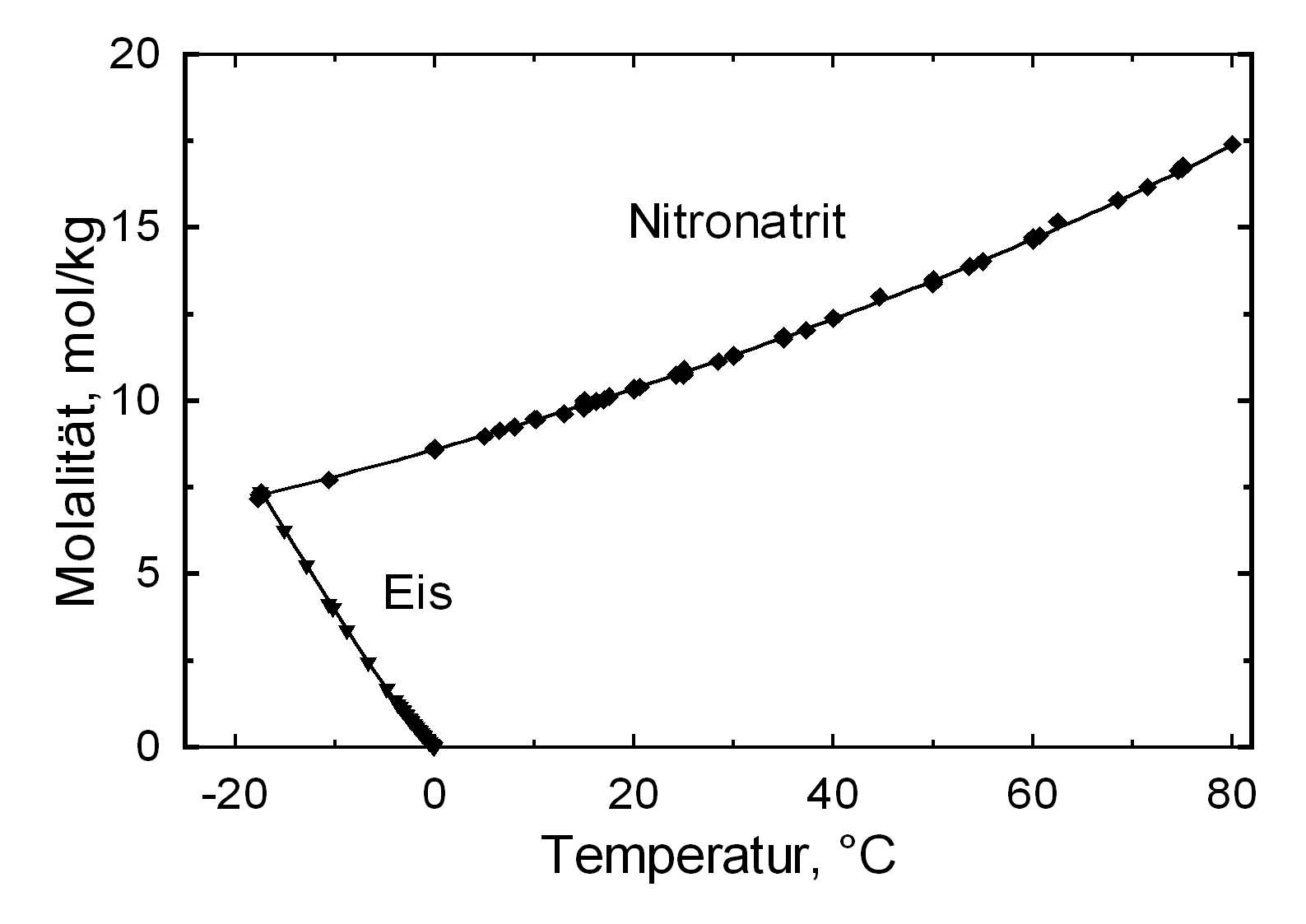
Solubility properties[edit]
Hygroscopicity[edit]
| 0°C | 10°C | 20°C | 30°C | 40°C | 50°C |
| 80.1%r.h. | 77.7%r.h. | 75.3%r.h. | 72.8%r.h. | 70.4%r.h. | 68.0%r.h. |
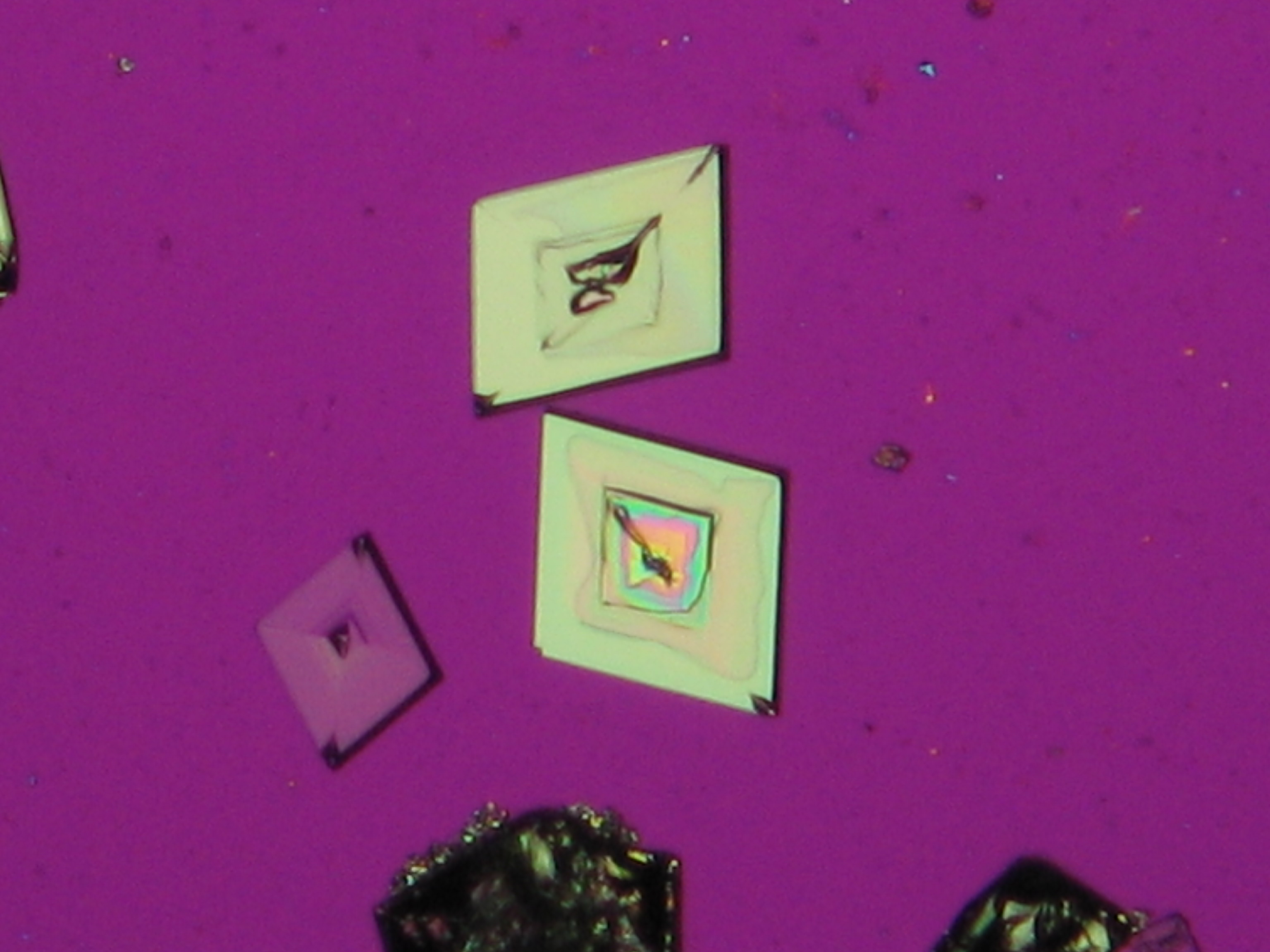
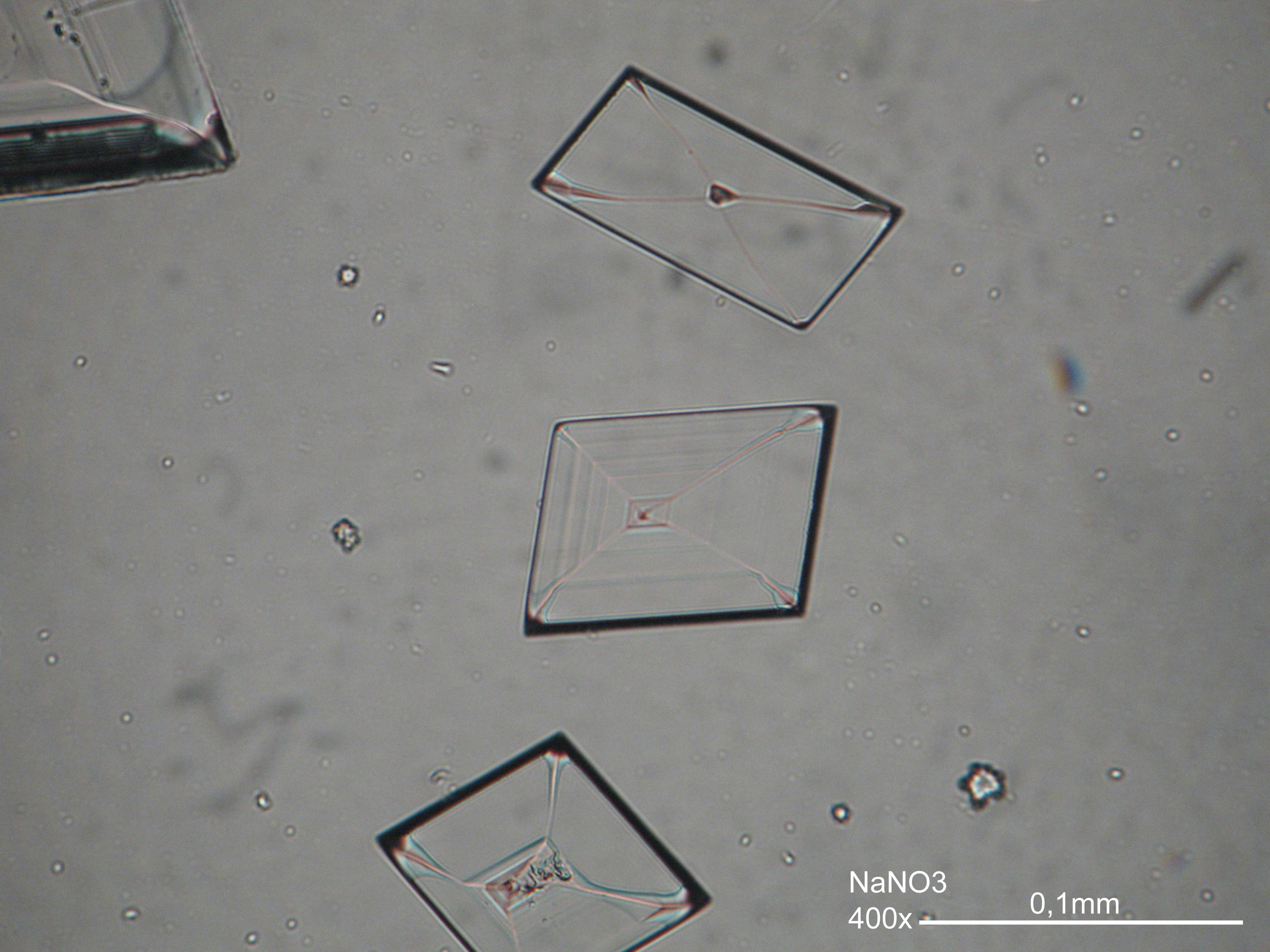
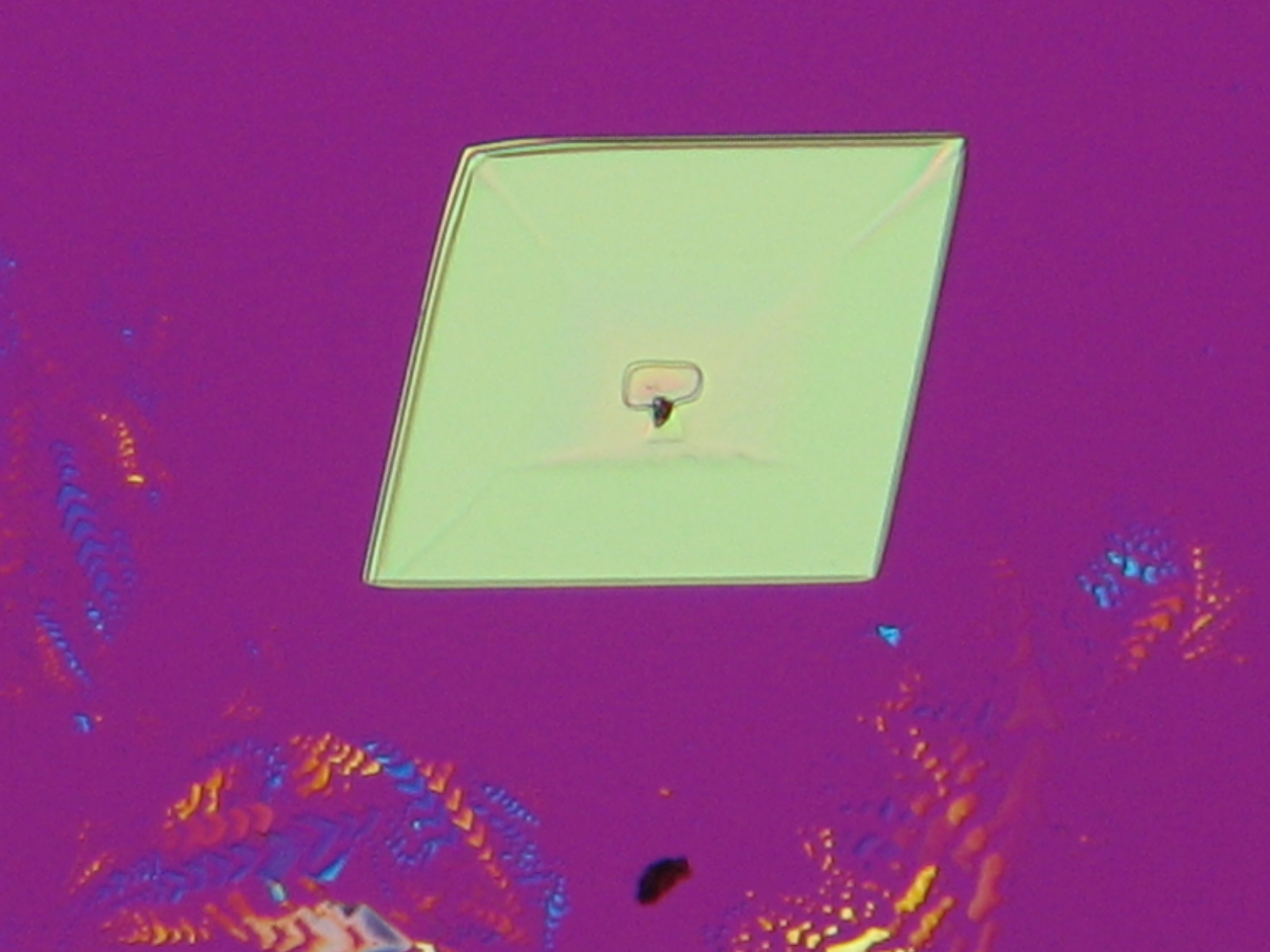
Under the polarising microscope[edit]
- crystallized on a glass slide
Weblinks
[edit]
- ↑ http://webmineral.com/data/Nitratine.shtml seen on 29.07.2010
- ↑ http://www.mindat.org/min-2916.html seen on 29.07.2010
Literature[edit]
| [Steiger etal: 2014] | Steiger, Michael; Charola A. Elena; Sterflinger, Katja (2014): Weathering and Deterioration. In:: Stone in Architecture, Siegesmund S. and Snethlage R., 10.1007/978-3-642-45155-3_4. |  |