Thenardite: Difference between revisions
No edit summary |
No edit summary |
||
| Line 5: | Line 5: | ||
|photo = [[File:HJS-Na2SO4-111703-02-10x.jpg|300px]] | |photo = [[File:HJS-Na2SO4-111703-02-10x.jpg|300px]] | ||
|mineralogical_Name = Thenardite | |mineralogical_Name = Thenardite | ||
|chemical_Name = Sodium | |chemical_Name = Sodium sulfate | ||
|Trivial_Name = Pyrotechnite | |Trivial_Name = Pyrotechnite | ||
|chemical_Formula = Na<sub>2</sub>SO<sub>4</sub> | |chemical_Formula = Na<sub>2</sub>SO<sub>4</sub> | ||
|Hydratforms = [[Mirabilite]] (Na<sub>2</sub>SO<sub>4</sub>•10H<sub>2</sub>O)<br> | |Hydratforms = [[Mirabilite]] (Na<sub>2</sub>SO<sub>4</sub>•10H<sub>2</sub>O)<br>Sodiumsulfate heptahydrate (Na<sub>2</sub>SO<sub>4</sub>•7H<sub>2</sub>O) | ||
|Crystal_System = orthorhombic | |Crystal_System = orthorhombic | ||
|Crystal_Structure = | |Crystal_Structure = | ||
|Deliqueszenzhumidity = 81 | |Deliqueszenzhumidity = 81.7% (25°C) | ||
|Solubility = 162 g/l | |Solubility = 162 g/l | ||
|Density = 2 | |Density = 2.689 g/cm³ | ||
|MolVolume = 53 | |MolVolume = 53.11 cm<sup>3</sup>/mol | ||
|Molweight = 142 | |Molweight = 142.04 g/mol | ||
|Transparency = transparent to translucent | |Transparency = transparent to translucent | ||
|Cleavage = perfect | |Cleavage = perfect | ||
|Crystal_Habit = | |Crystal_Habit = | ||
|Twinning = | |Twinning = | ||
|Refractive_Indices = n<sub>x</sub> = 1 | |Refractive_Indices = n<sub>x</sub> = 1.468<br> n<sub>y</sub> = 1.473<br> n<sub>z</sub> = 1.483 | ||
|Birefringence = Δ = 0 | |Birefringence = Δ = 0.015 | ||
|optical_Orientation = positive | |optical_Orientation = positive | ||
|Pleochroism = | |Pleochroism = | ||
| Line 32: | Line 32: | ||
Authors: [[user:Hschwarz|Hans-Jürgen Schwarz ]], Michael Steiger, [[user:TMueller|Tim Müller]] <br> | Authors: [[user:Hschwarz|Hans-Jürgen Schwarz ]], Michael Steiger, [[user:TMueller|Tim Müller]] <br> | ||
English translation: [[user:MAngeli|Matthieu Angeli]] <br> | English translation: [[user:MAngeli|Matthieu Angeli]] <br> | ||
back to [[ | back to [[Sulfate]] | ||
= Sodium | = Sodium sulfate and thenardite = | ||
__TOC__ | __TOC__ | ||
== Abstract == | == Abstract == | ||
Sodium | Sodium sulfate and its phases thenardite, mirabilite and the heptahydrate, whose importance in the production of damage has been identified, are described. | ||
== Introduction == | == Introduction == | ||
| Line 46: | Line 46: | ||
== Occurence == | == Occurence == | ||
Both thenardite like [[mirabilite]] appear as natural minerals. Sodium | Both thenardite like [[mirabilite]] appear as natural minerals. Sodium sulfate appears in Nature in mineral waters in the form of double salts, as deposits of former salt lakes. Knowledge of the hydrated sodium sulfate dates back to the 16th Century. Its first description has been written by Glauber in 1658, in which he described it as "sal mirable". It is also quite common to read the name "Glauber's salt" for [[mirabilite]] in the literature. | ||
== Information on the origin and formation of thenardite / mirabilite in monuments == | == Information on the origin and formation of thenardite / mirabilite in monuments == | ||
With the entry of materials that contain soluble sodium compounds, the mineral system of a monument may create sodium | With the entry of materials that contain soluble sodium compounds, the mineral system of a monument may create sodium sulfate as salt efflorescence when acting with various sources of sulfate such as for example sulphurous gases or contaminated air. Cement exhibits a high content of sodium ions, as they are allowed by the German Standardization Institute to contain up to 0.5 % of soluble alkalis. This means that 100 kg of Portland cement containing only 0.1% soluble Na<sub>2</sub>O can form 520g of [[Mirabilite]] when in contact with air containing sulfuric acid [calculation from Arnold/Zehnder 1991]. Sodium ions can also enter into monuments from a plethora of cleaning materials and especially older restoration products (such as water glass). Ground water and surface water are also a possible source of Na<sup>+</sup>-ions. Road salt consists to a large part of slightly soluble [[Halite|sodium chloride]]. Finally, in the coastal areas, sea water is also a significant source of [[Halite|NaCl]]. | ||
== Solubility behavior == | == Solubility behavior == | ||
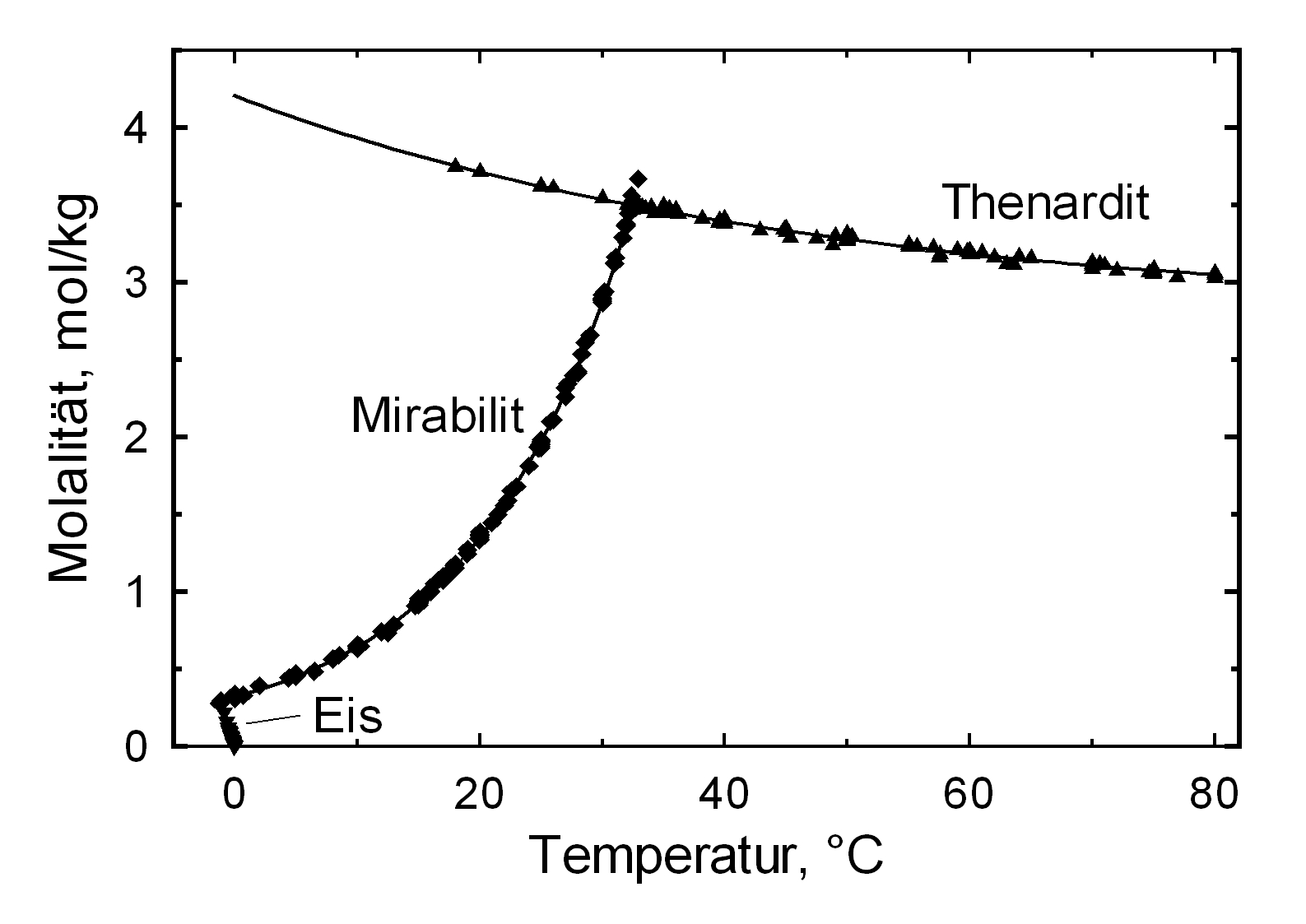
[[file:Na2SO4_sol.jpg|thumb|350px|right|'''Abbildung 1''': Solubility of Na2SO4 in water, Graph: M. Steiger]]<br> | [[file:Na2SO4_sol.jpg|thumb|350px|right|'''Abbildung 1''': Solubility of Na2SO4 in water, Graph: M. Steiger]]<br> | ||
The structures of both thenardite and [[mirabilite]] belong to the group of easily soluble salts and therefore easily mobilizable (see Table [[hygroscopicity of the salts and ERH]]). The solubility of sodium | The structures of both thenardite and [[mirabilite]] belong to the group of easily soluble salts and therefore easily mobilizable (see Table [[hygroscopicity of the salts and ERH]]). The solubility of sodium sulfate is highly dependent on temperature. For this reason, a rapid drop of temperature is highly likely to yield very high supersaturation and salt crystallization.<br> | ||
== Hygroscopicity == | == Hygroscopicity == | ||
| Line 83: | Line 83: | ||
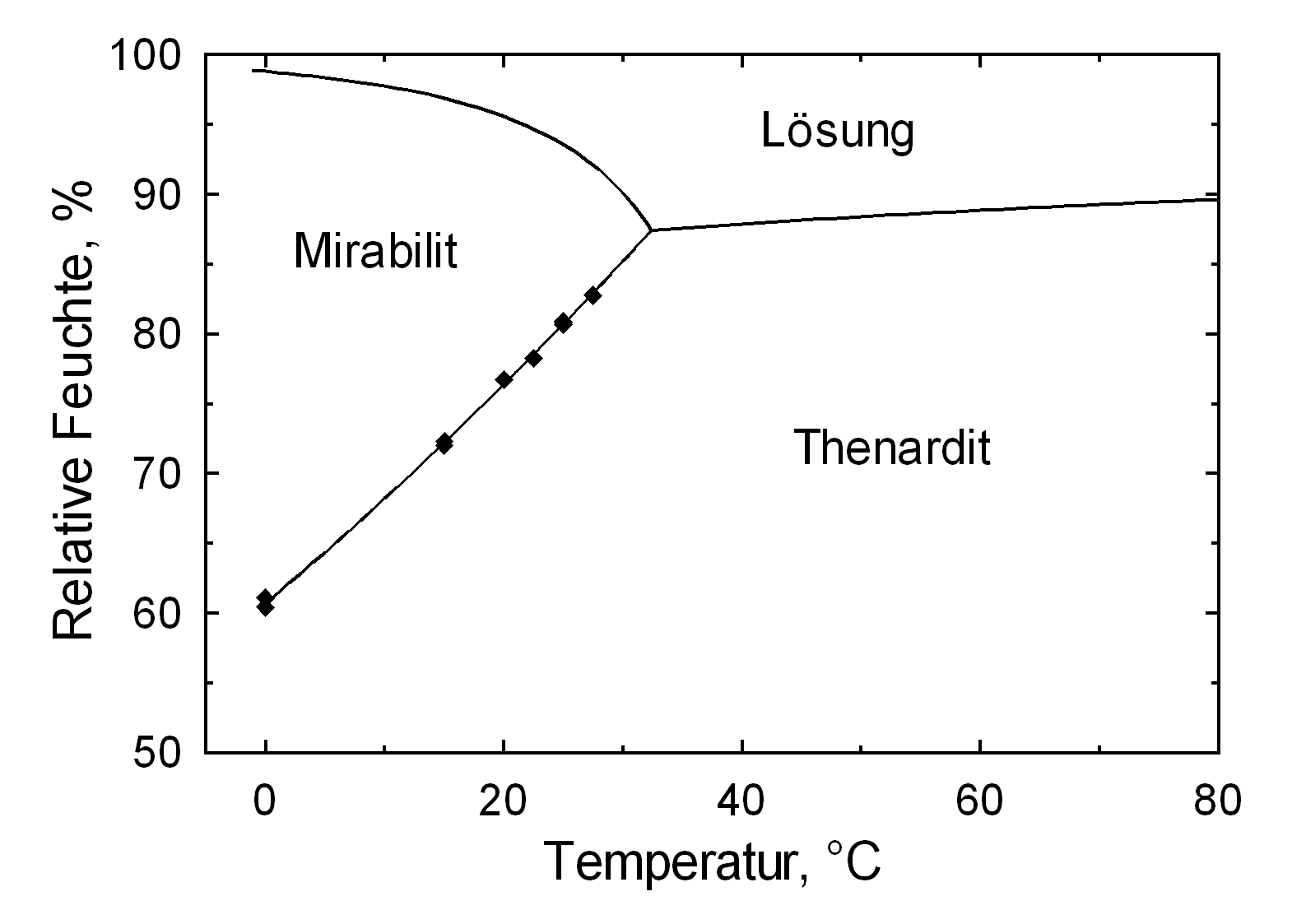
[[file:Deliqueszenz Mirabilit, Thenardit .JPG|thumb|350px|right|'''Abbildung 3''': Deliquescence points of pure salts thenardite and mirabilite <bib id=Arnold.etal:1991/>]] | [[file:Deliqueszenz Mirabilit, Thenardit .JPG|thumb|350px|right|'''Abbildung 3''': Deliquescence points of pure salts thenardite and mirabilite <bib id=Arnold.etal:1991/>]] | ||
The table below shows additional information for estimating the hygroscopicity of sodium | The table below shows additional information for estimating the hygroscopicity of sodium sulfate for the sorption behavior of pure salt and the mixture with [[Halite]] at different relative humidities: | ||
<br> | <br> | ||
| Line 107: | Line 107: | ||
== Crystallization pressure == | == Crystallization pressure == | ||
When crystallizing from an aqueous solution, the crystallization pressure of thenardite lies in the 29 | When crystallizing from an aqueous solution, the crystallization pressure of thenardite lies in the 29.2-34.5 N/mm<sup>2</sup> range. These values are higher that those calculated for other building-damaging salts <bib id=Winkler:1975/>. | ||
== Hydration behavior == | == Hydration behavior == | ||
| Line 127: | Line 127: | ||
|- | |- | ||
|bgcolor = "#F0F0F0" align=center| '''rel. Feuchte %''' | |bgcolor = "#F0F0F0" align=center| '''rel. Feuchte %''' | ||
|bgcolor = "#F0F0F0" align=center| '''20 | |bgcolor = "#F0F0F0" align=center| '''20.0 °C''' | ||
|bgcolor = "#F0F0F0" align=center| '''25 | |bgcolor = "#F0F0F0" align=center| '''25.0 °C''' | ||
|bgcolor = "#F0F0F0" align=center| '''30 | |bgcolor = "#F0F0F0" align=center| '''30.0 °C''' | ||
|- | |- | ||
|bgcolor = "#F7F7F7" align=center| '''100''' | |bgcolor = "#F7F7F7" align=center| '''100''' | ||
|bgcolor = "#FFFFEO" align="center"| 48 | |bgcolor = "#FFFFEO" align="center"| 48.9 N/mm<sup>2</sup> | ||
|bgcolor = "#FFFFEO" align="center"| 40 | |bgcolor = "#FFFFEO" align="center"| 40.5 N/mm<sup>2</sup> | ||
|bgcolor = "#FFFFEO" align="center"| 28 | |bgcolor = "#FFFFEO" align="center"| 28.9 N/mm<sup>2</sup> | ||
|- | |- | ||
|bgcolor = "#F7F7F7" align=center| '''95,0''' | |bgcolor = "#F7F7F7" align=center| '''95,0''' | ||
|bgcolor = "#FFFFEO" align="center"| 41 | |bgcolor = "#FFFFEO" align="center"| 41.3 N/mm<sup>2</sup> | ||
|bgcolor = "#FFFFEO" align="center"| 32 | |bgcolor = "#FFFFEO" align="center"| 32.7 N/mm<sup>2</sup> | ||
|bgcolor = "#FFFFEO" align="center"| 23 | |bgcolor = "#FFFFEO" align="center"| 23.3 N/mm<sup>2</sup> | ||
|- | |- | ||
|bgcolor = "#F7F7F7" align="center"| '''90,0''' | |bgcolor = "#F7F7F7" align="center"| '''90,0''' | ||
|bgcolor = "#FFFFEO" align="center"| 33 | |bgcolor = "#FFFFEO" align="center"| 33.5 N/mm<sup>2</sup> | ||
|bgcolor = "#FFFFEO" align="center"| 24 | |bgcolor = "#FFFFEO" align="center"| 24.9 N/mm<sup>2</sup> | ||
|bgcolor = "#FFFFEO" align="center"| 13 | |bgcolor = "#FFFFEO" align="center"| 13.7 N/mm<sup>2</sup> | ||
|- | |- | ||
|bgcolor = "#F7F7F7" align="center"| '''85,0''' | |bgcolor = "#F7F7F7" align="center"| '''85,0''' | ||
|bgcolor = "#FFFFEO" align="center"| 25 | |bgcolor = "#FFFFEO" align="center"| 25.5 N/mm<sup>2</sup> | ||
|bgcolor = "#FFFFEO" align="center"| 16 | |bgcolor = "#FFFFEO" align="center"| 16.0 N/mm<sup>2</sup> | ||
|bgcolor = "#FFFFEO" align="center"| 5 | |bgcolor = "#FFFFEO" align="center"| 5.1 N/mm<sup>2</sup> | ||
|- | |- | ||
|bgcolor = "#F7F7F7" align="center"| '''80,0''' | |bgcolor = "#F7F7F7" align="center"| '''80,0''' | ||
|bgcolor = "#FFFFEO" align="center"| 16 | |bgcolor = "#FFFFEO" align="center"| 16.4 N/mm<sup>2</sup> | ||
|bgcolor = "#FFFFEO" align="center"| 7 | |bgcolor = "#FFFFEO" align="center"| 7.8 N/mm<sup>2</sup> | ||
|bgcolor = "#FFFFEO" align="center"| 0 | |bgcolor = "#FFFFEO" align="center"| 0.0 | ||
|- | |- | ||
|bgcolor = "#F7F7F7" align="center"| '''75 | |bgcolor = "#F7F7F7" align="center"| '''75.0''' | ||
|bgcolor = "#FFFFEO" align="center"| 6 | |bgcolor = "#FFFFEO" align="center"| 6.7 N/mm<sup>2</sup> | ||
|bgcolor = "#FFFFEO" align="center"| 0 | |bgcolor = "#FFFFEO" align="center"| 0.0 | ||
|bgcolor = "#FFFFEO" align="center"| - | |bgcolor = "#FFFFEO" align="center"| - | ||
|} | |} | ||
| Line 171: | Line 171: | ||
'''Laboruntersuchung:'''<br>Durch mikroskopische Beobachtungen des Lösungsverhaltens sind die gute Wasserlöslichkeit und Ethanolunlöslichkeit zu verifizieren. Thenardit und [[Mirabilit]] besitzen keine morphologische Charakterisitka, die bei einfachen Rekristallisationsversuchen zur Identifizierung beitragen können. Vielmehr ist eine große Bandbreite unterschiedlichster Erscheinungsformen beobachtbar.<br> | '''Laboruntersuchung:'''<br>Durch mikroskopische Beobachtungen des Lösungsverhaltens sind die gute Wasserlöslichkeit und Ethanolunlöslichkeit zu verifizieren. Thenardit und [[Mirabilit]] besitzen keine morphologische Charakterisitka, die bei einfachen Rekristallisationsversuchen zur Identifizierung beitragen können. Vielmehr ist eine große Bandbreite unterschiedlichster Erscheinungsformen beobachtbar.<br> | ||
'''Brechungsindizes:''' n<sub>x</sub> = 1 | '''Brechungsindizes:''' n<sub>x</sub> = 1.468; n<sub>y</sub> =1,473; n<sub>z</sub> =1.483<br>'''Doppelbrechung''': Δ = 0.015<br>'''Kristallklass'''e: orthorhombisch<br> | ||
<br> | <br> | ||
| Line 198: | Line 198: | ||
|- | |- | ||
|bgcolor = "#F7F7F7"|'''[[Astrakanit|Bloedit]]''' Na<sub>2</sub>Mg(SO<sub>4</sub>)<sub>2</sub> • 6H<sub>2</sub>0 | |bgcolor = "#F7F7F7"|'''[[Astrakanit|Bloedit]]''' Na<sub>2</sub>Mg(SO<sub>4</sub>)<sub>2</sub> • 6H<sub>2</sub>0 | ||
|bgcolor = "#FFFFEO"| alle Indizes >1 | |bgcolor = "#FFFFEO"| alle Indizes >1.48 / keine anormalen Interferenzfarben / schiefe Auslöschung / optisch negativ orientiert. | ||
|- | |- | ||
|bgcolor = "#F7F7F7"| '''[[Aphthitalit|Glaserit]]''' K<sub>3</sub>Na(SO<sub>4</sub>)<sub>2</sub> | |bgcolor = "#F7F7F7"| '''[[Aphthitalit|Glaserit]]''' K<sub>3</sub>Na(SO<sub>4</sub>)<sub>2</sub> | ||
|bgcolor = "#FFFFEO"| alle Indizes >1 | |bgcolor = "#FFFFEO"| alle Indizes >1.48 / keine anormalen Interferenzfarben/schiefe Auslöschung | ||
|- | |- | ||
|bgcolor = "#F7F7F7"| '''[[Arcanit]]''' K<sub>2</sub>SO<sub>4</sub> | |bgcolor = "#F7F7F7"| '''[[Arcanit]]''' K<sub>2</sub>SO<sub>4</sub> | ||
|bgcolor = "#FFFFEO"| alle Indizes >1 | |bgcolor = "#FFFFEO"| alle Indizes >1.48 / keine anormalen Interferenzfarben | ||
|- | |- | ||
|bgcolor = "#F7F7F7"| '''[[Magnesiumformiat]]''' Mg(HCO<sub>2</sub>)<sub>2</sub> • 2H<sub>2</sub>O | |bgcolor = "#F7F7F7"| '''[[Magnesiumformiat]]''' Mg(HCO<sub>2</sub>)<sub>2</sub> • 2H<sub>2</sub>O | ||
Revision as of 16:48, 20 December 2011
<bibimport/>
| Thenardite[1][2] | |
| |
| Mineralogical name | Thenardite |
| Chemical name | Sodium sulfate |
| Trivial name | Pyrotechnite |
| Chemical formula | Na2SO4 |
| Other forms | Mirabilite (Na2SO4•10H2O) Sodiumsulfate heptahydrate (Na2SO4•7H2O) |
| Crystal system | orthorhombic |
| Crystal structure | |
| Deliquescence humidity 20°C | 81.7% (25°C) |
| Solubility (g/l) at 20°C | 162 g/l |
| Density (g/cm³) | 2.689 g/cm³ |
| Molar volume | 53.11 cm3/mol |
| Molar weight | 142.04 g/mol |
| Transparency | transparent to translucent |
| Cleavage | perfect |
| Crystal habit | |
| Twinning | |
| Phase transition | |
| Chemical behavior | |
| Comments | soluble in water and glycerin, not soluble in pure alcohol |
| Crystal Optics | |
| Refractive Indices | nx = 1.468 ny = 1.473 nz = 1.483 |
| Birefringence | Δ = 0.015 |
| Optical Orientation | positive |
| Pleochroism | |
| Dispersion | |
| Used Literature | |
| {{{Literature}}} | |
Authors: Hans-Jürgen Schwarz , Michael Steiger, Tim Müller
English translation: Matthieu Angeli
back to Sulfate
Sodium sulfate and thenardite[edit]
Abstract[edit]
Sodium sulfate and its phases thenardite, mirabilite and the heptahydrate, whose importance in the production of damage has been identified, are described.
Introduction[edit]
Occurence[edit]
Both thenardite like mirabilite appear as natural minerals. Sodium sulfate appears in Nature in mineral waters in the form of double salts, as deposits of former salt lakes. Knowledge of the hydrated sodium sulfate dates back to the 16th Century. Its first description has been written by Glauber in 1658, in which he described it as "sal mirable". It is also quite common to read the name "Glauber's salt" for mirabilite in the literature.
Information on the origin and formation of thenardite / mirabilite in monuments[edit]
With the entry of materials that contain soluble sodium compounds, the mineral system of a monument may create sodium sulfate as salt efflorescence when acting with various sources of sulfate such as for example sulphurous gases or contaminated air. Cement exhibits a high content of sodium ions, as they are allowed by the German Standardization Institute to contain up to 0.5 % of soluble alkalis. This means that 100 kg of Portland cement containing only 0.1% soluble Na2O can form 520g of Mirabilite when in contact with air containing sulfuric acid [calculation from Arnold/Zehnder 1991]. Sodium ions can also enter into monuments from a plethora of cleaning materials and especially older restoration products (such as water glass). Ground water and surface water are also a possible source of Na+-ions. Road salt consists to a large part of slightly soluble sodium chloride. Finally, in the coastal areas, sea water is also a significant source of NaCl.
Solubility behavior[edit]
The structures of both thenardite and mirabilite belong to the group of easily soluble salts and therefore easily mobilizable (see Table hygroscopicity of the salts and ERH). The solubility of sodium sulfate is highly dependent on temperature. For this reason, a rapid drop of temperature is highly likely to yield very high supersaturation and salt crystallization.
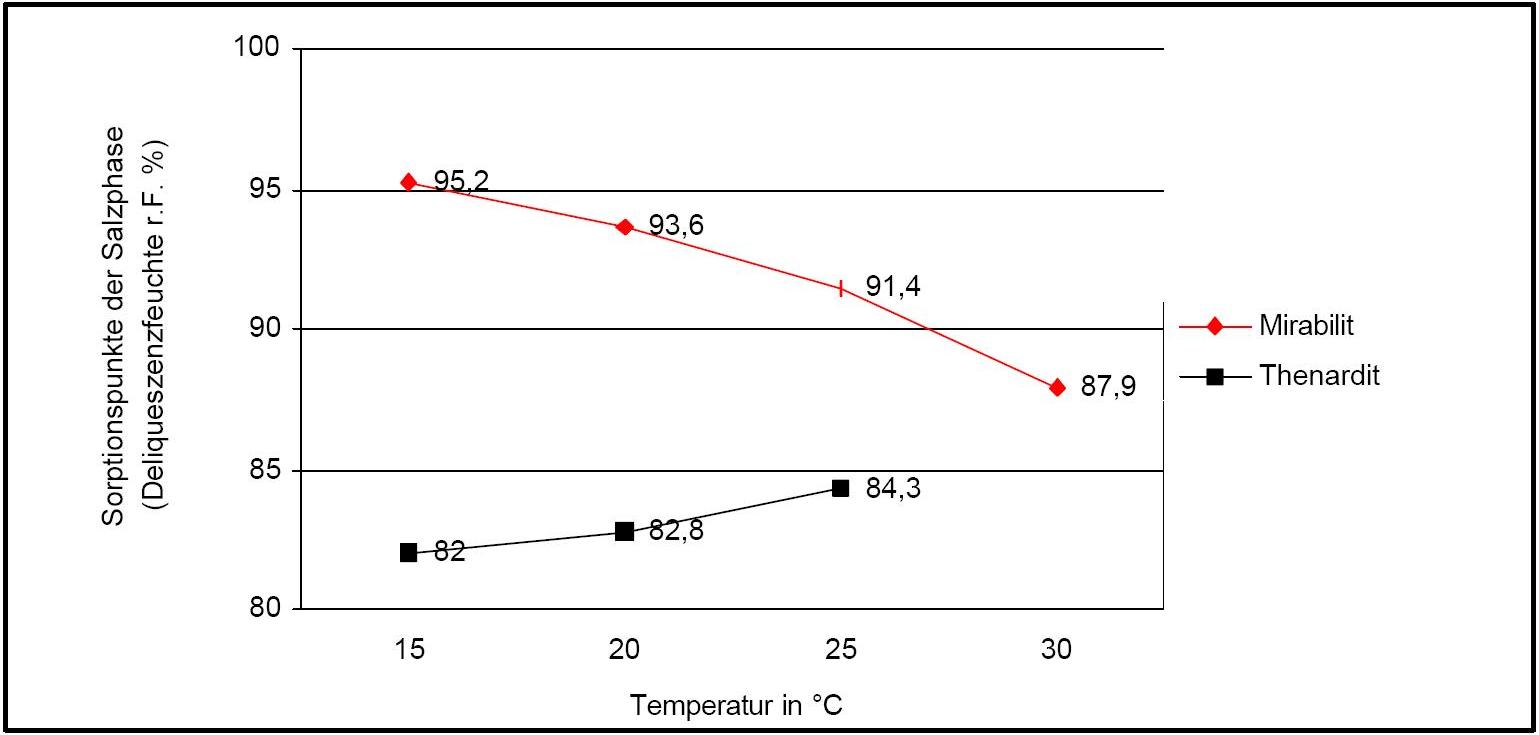
Hygroscopicity[edit]
The temperature effect on the deliquescence points of thenardite and mirabilite is shown below. The striking features here are the opposite curve transitions. In the presence of other ions (in salt mixtures), the parameters of the equilibrium moisture content as well as the necessary temperature and humidity conditions for recrystallization change significantly. The following table shows experimental data of equilibrium moisture for different salt mixtures at different temperatures.It turns out that all the values of equilibrium moisture content are lower than those of pure salt mirabilite (see table equilibrium moisture content as a function of temperature).
| MgSO4 | Ca(NO3)2 | KNO3 | |
| Na2SO4 • 10H2O | 87(21°C) | 74 (21°C) | 81(21°C) |
Water vapor sorption:
Author: Arnold, Andreas; Zehnder, Konrad

The table below shows additional information for estimating the hygroscopicity of sodium sulfate for the sorption behavior of pure salt and the mixture with Halite at different relative humidities:
| Lagerungsfeuchte | 87% r.F. | 81% r.F. | 79% r.F. |
| Na2SO4 | 79 | 0 | 0 |
| Na2SO4+NaCl (1:1 molare Mischung) | 157 | 32 | 15 |
Crystallization pressure[edit]
When crystallizing from an aqueous solution, the crystallization pressure of thenardite lies in the 29.2-34.5 N/mm2 range. These values are higher that those calculated for other building-damaging salts [Winkler:1975]Title: Stone: Properties, Durability in Man´s Environment
Author: Winkler, Erhard M. .
.
Hydration behavior[edit]
The Na2SO4 – H2O system:
The only stable forms are the decahydrate (Mirabilite) and the anhydrite (Thenardite). The generation of mirabilite by recrystallization of the salt from an aqueous supersaturated solution occurs at 32.4°C. In particular, the transition from thenardite to mirabilite and the incorporation of 10 water molecules in the crystal lattice causes a volume expansion of 320%. This transition happening at a relatively low temperature (32-35°C), the damage caused by this salt is highly dependent on the temperature and thus on the environment. This temperature range is given as a guide, as this transition could happen for example at 25°C at 80% relative humidity, or even at 0°C at 60.7% relative humidity [information from Gmelin]. Because of this strong dependence on the environmental parameters, an estimate of the damage caused on buildings by crystallization and hydration of sodium sulfate are very difficult to obtain.
The importance of the heptahydrate in the damage process[edit]
Hydration pressure[edit]
Der Hydratationsdruck, der beim Übergang von Thenardit zu Mirabilit aufgebaut wird, ist stark abhängig von den bestehenden Luftfeuchte- und Temperatur-verhältnissen, was in der nachstehenden Tabelle verdeutlicht ist:
| rel. Feuchte % | 20.0 °C | 25.0 °C | 30.0 °C |
| 100 | 48.9 N/mm2 | 40.5 N/mm2 | 28.9 N/mm2 |
| 95,0 | 41.3 N/mm2 | 32.7 N/mm2 | 23.3 N/mm2 |
| 90,0 | 33.5 N/mm2 | 24.9 N/mm2 | 13.7 N/mm2 |
| 85,0 | 25.5 N/mm2 | 16.0 N/mm2 | 5.1 N/mm2 |
| 80,0 | 16.4 N/mm2 | 7.8 N/mm2 | 0.0 |
| 75.0 | 6.7 N/mm2 | 0.0 | - |
Die Volumenveränderung, die beim Phasenübergang stattfindet, ist mit ca. 320% anzugeben [Sperling.etal:1980]Title: Salt Weathering on Arid Environment, I. Theoretical ConsiderationsII. Laboratory Studies
Author: Sperling, C.H.B.and Cooke, R.U. .
.
Analytical detection[edit]
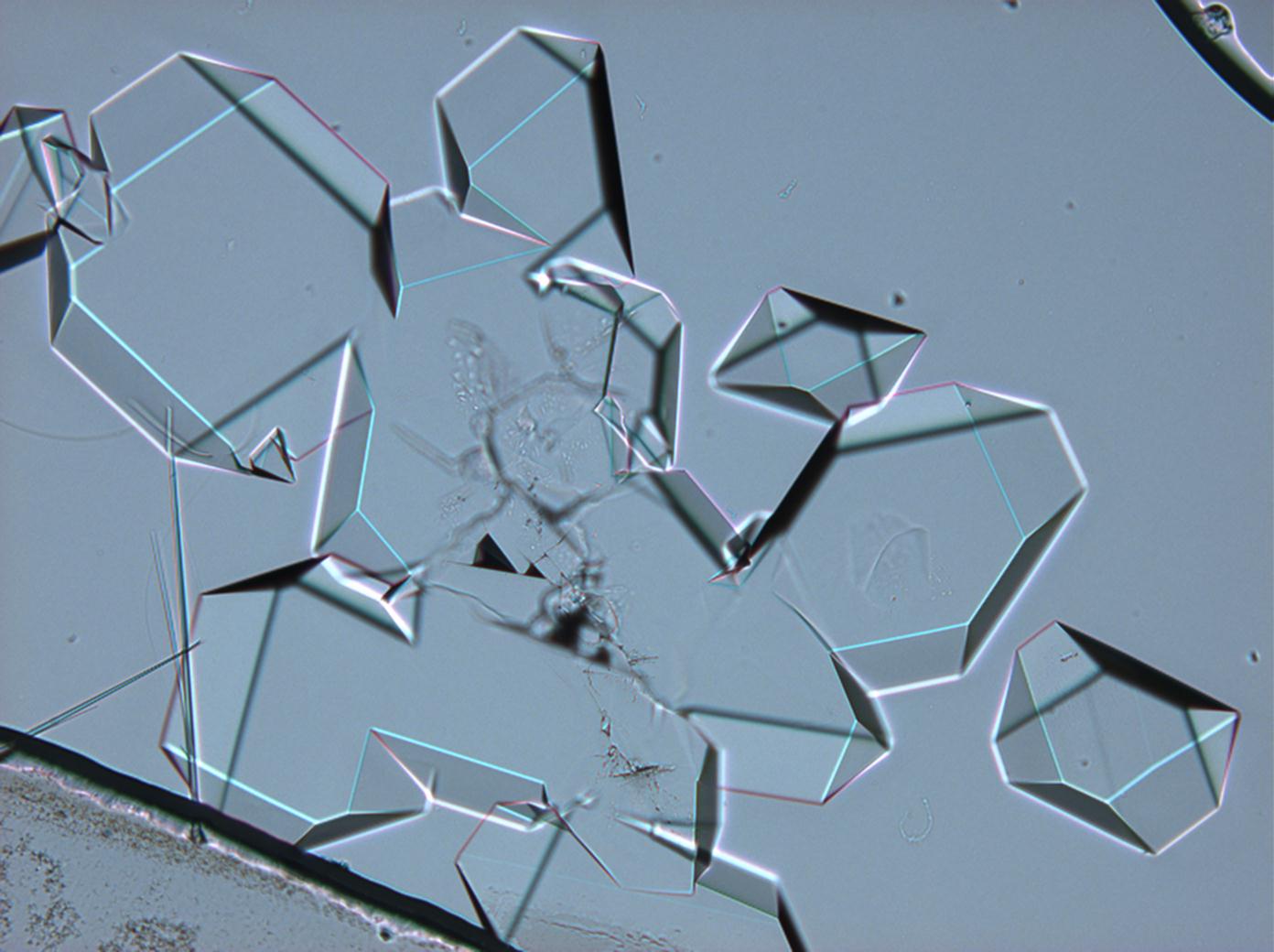
Microscopy
[edit]
Laboruntersuchung:
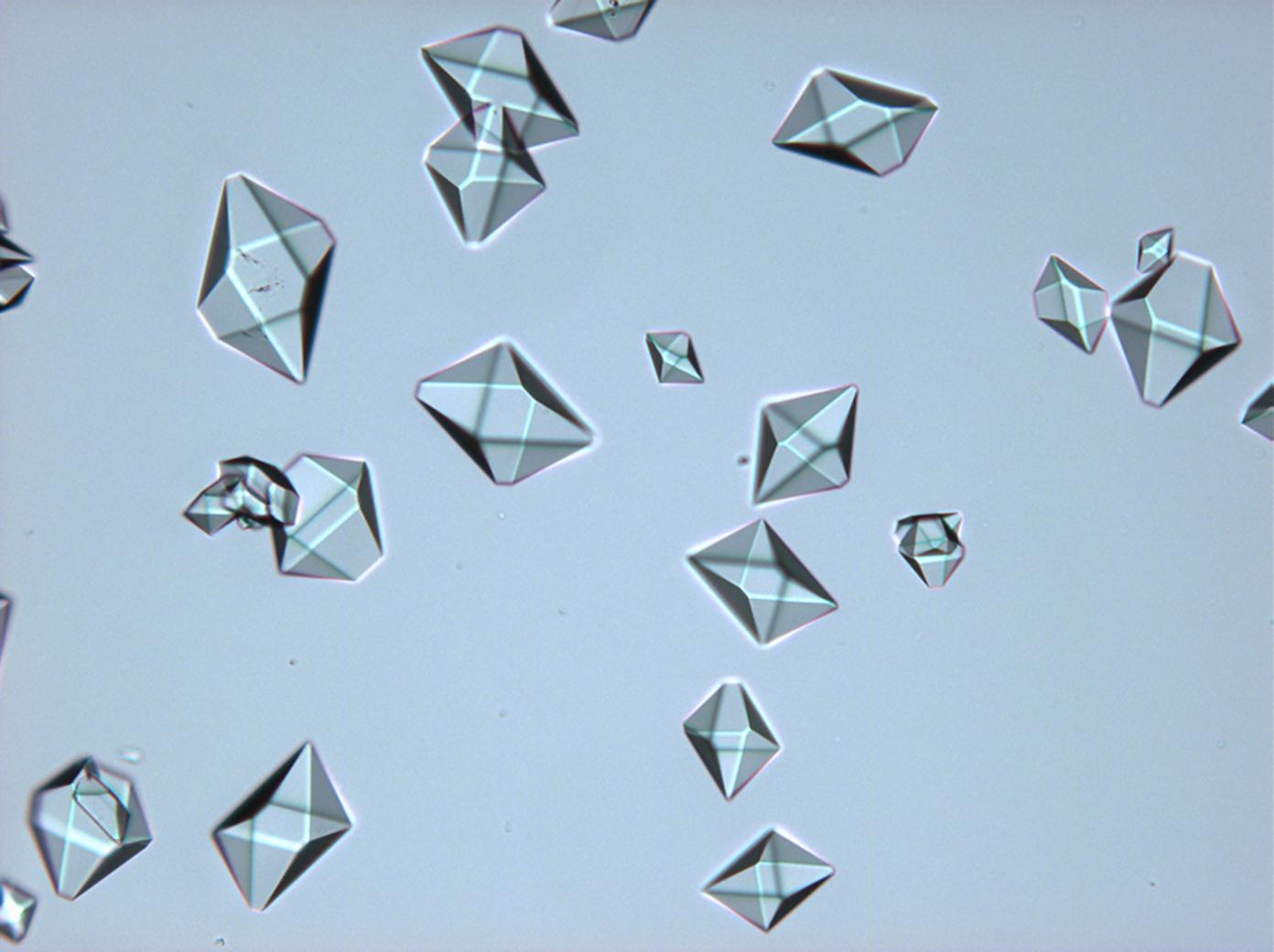
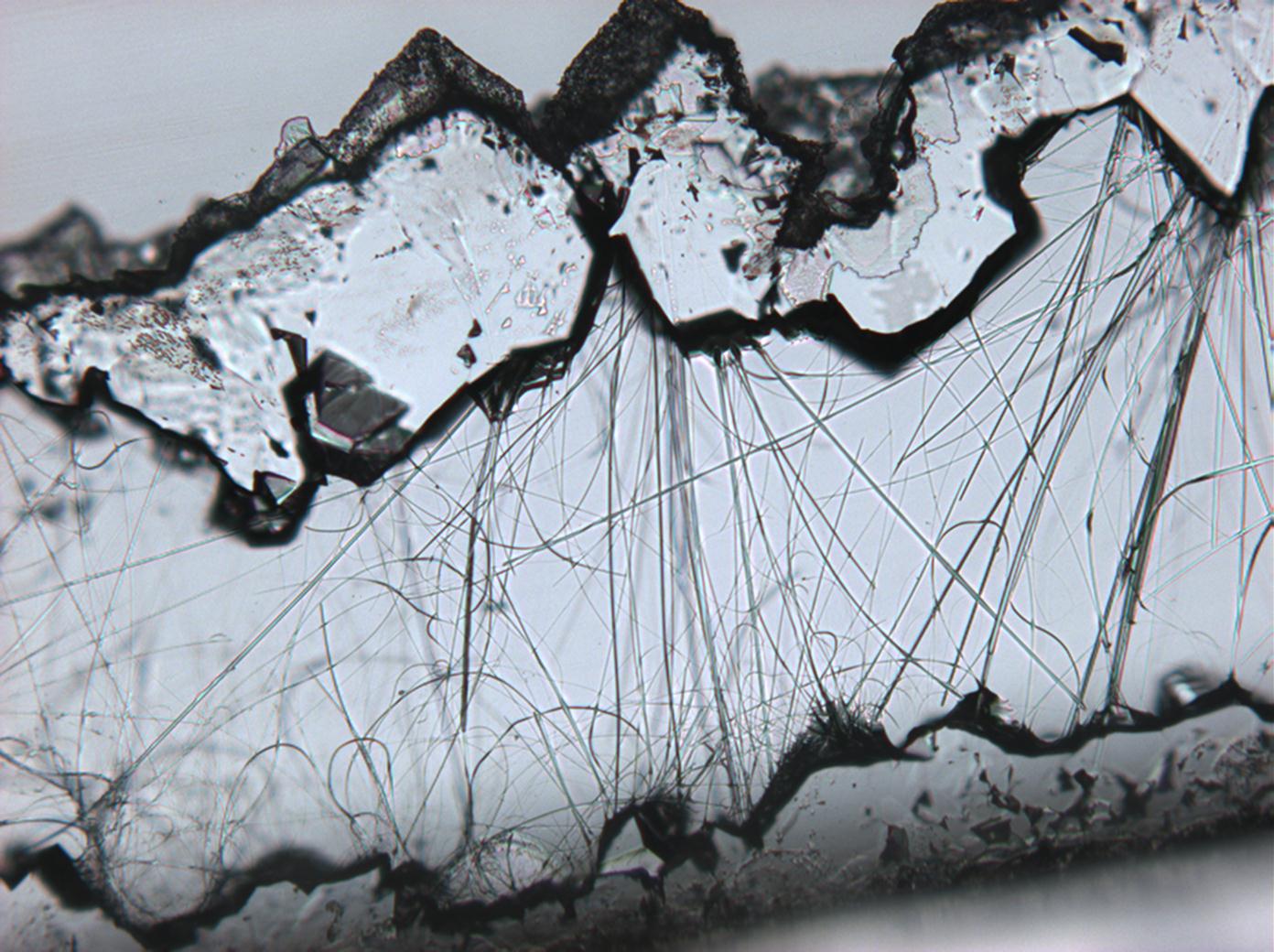
Durch mikroskopische Beobachtungen des Lösungsverhaltens sind die gute Wasserlöslichkeit und Ethanolunlöslichkeit zu verifizieren. Thenardit und Mirabilit besitzen keine morphologische Charakterisitka, die bei einfachen Rekristallisationsversuchen zur Identifizierung beitragen können. Vielmehr ist eine große Bandbreite unterschiedlichster Erscheinungsformen beobachtbar.
Brechungsindizes: nx = 1.468; ny =1,473; nz =1.483
Doppelbrechung: Δ = 0.015
Kristallklasse: orthorhombisch
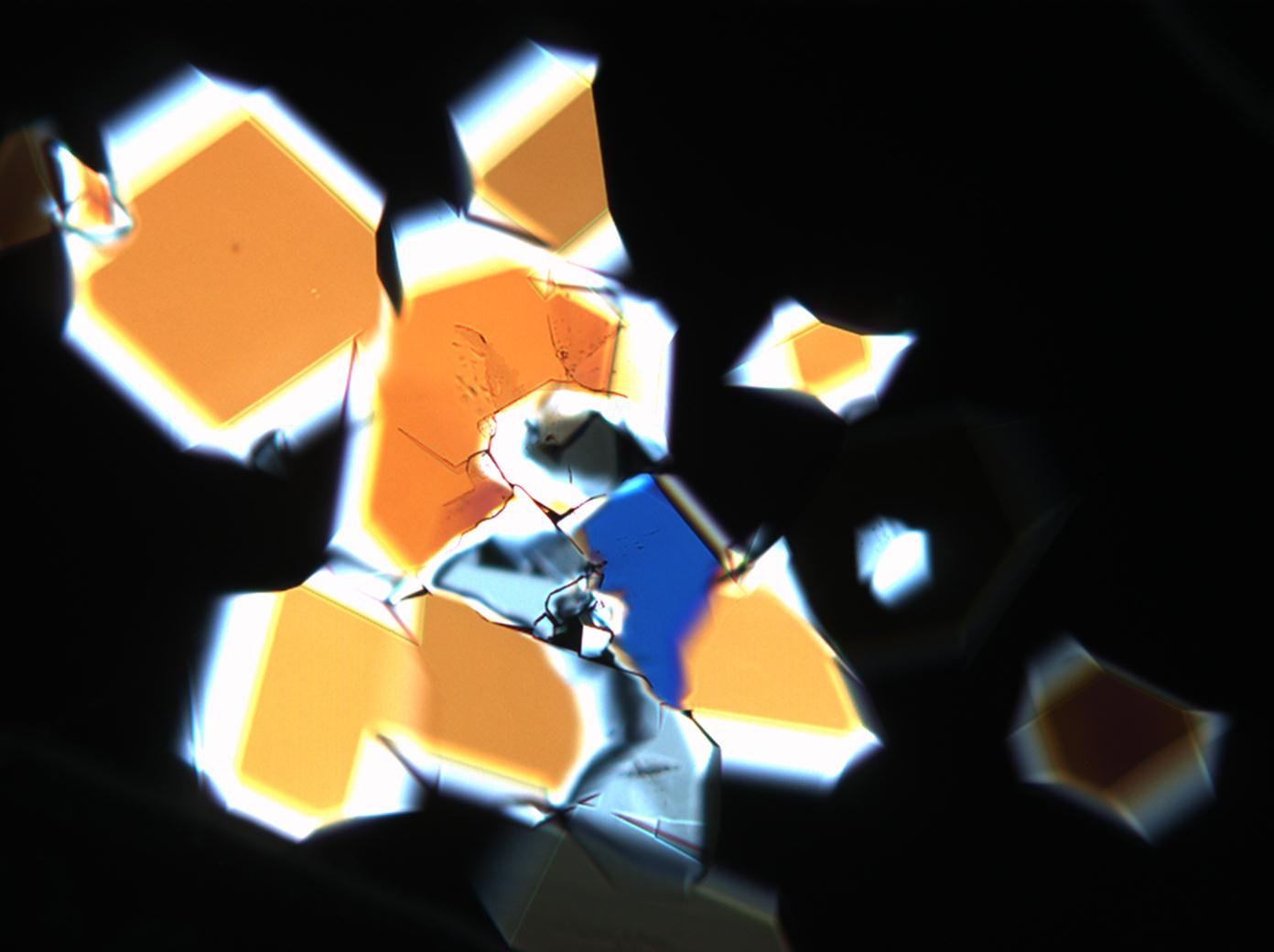
Polarisationsmikroskopische Investigation:
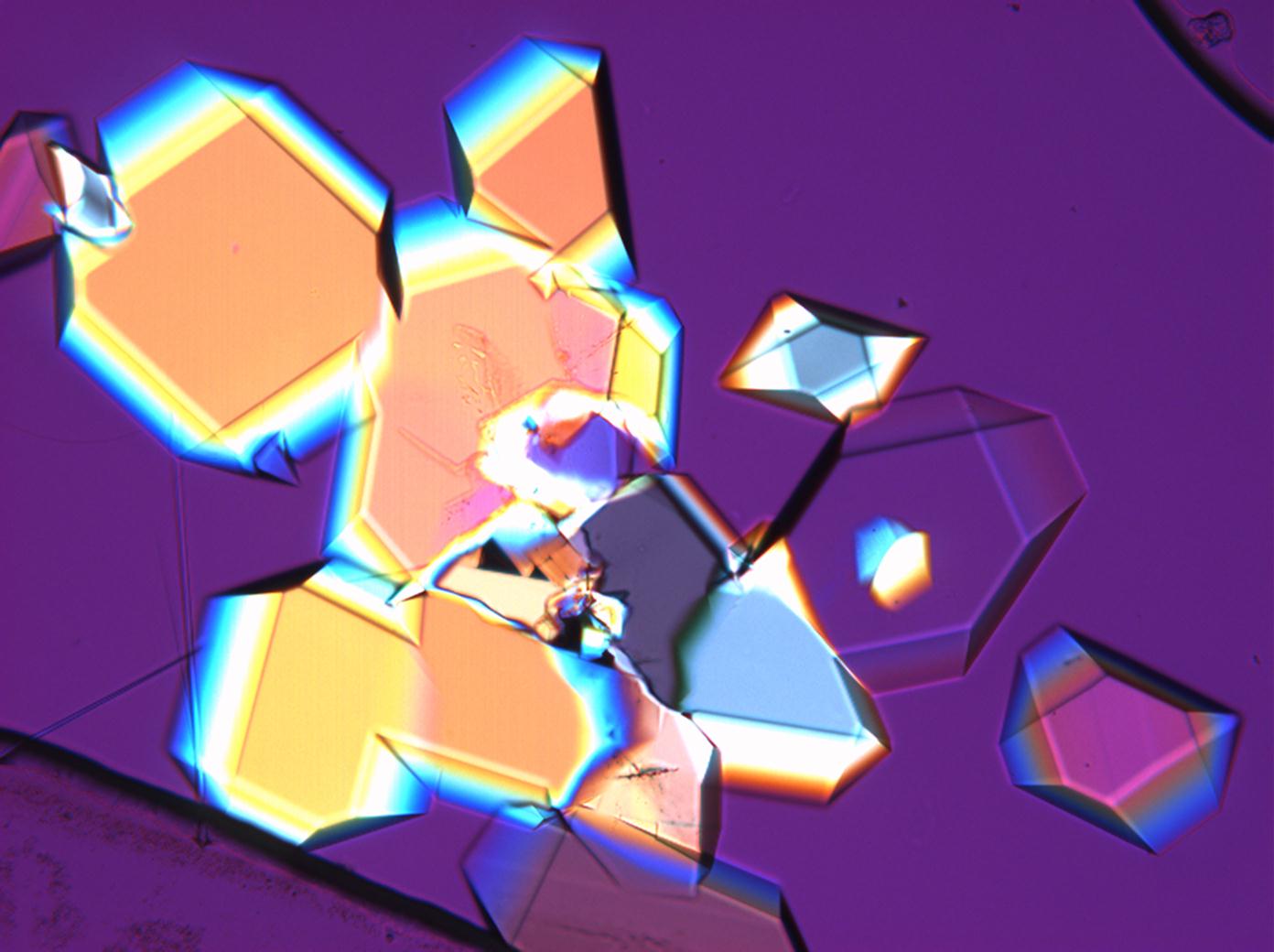
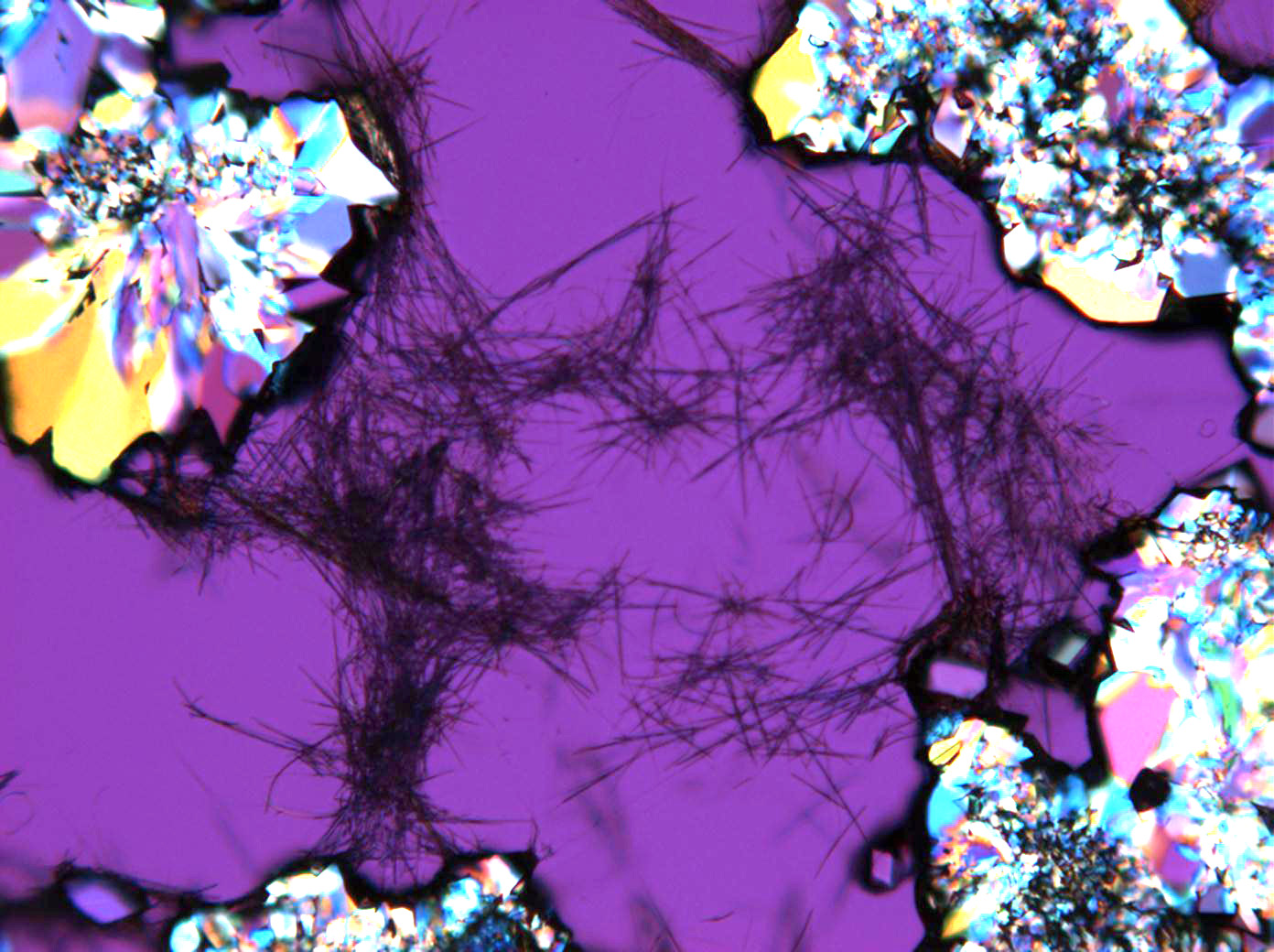
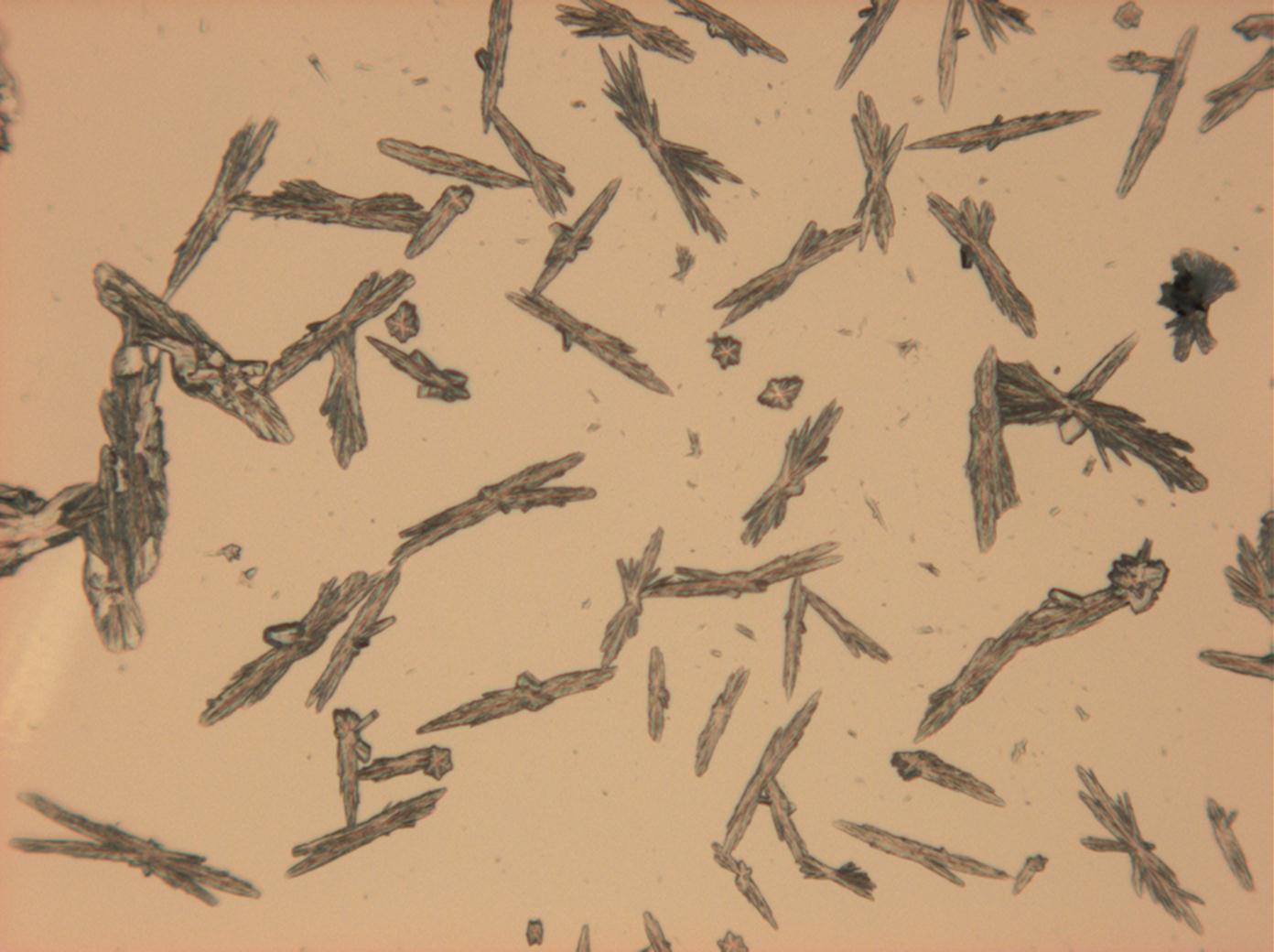
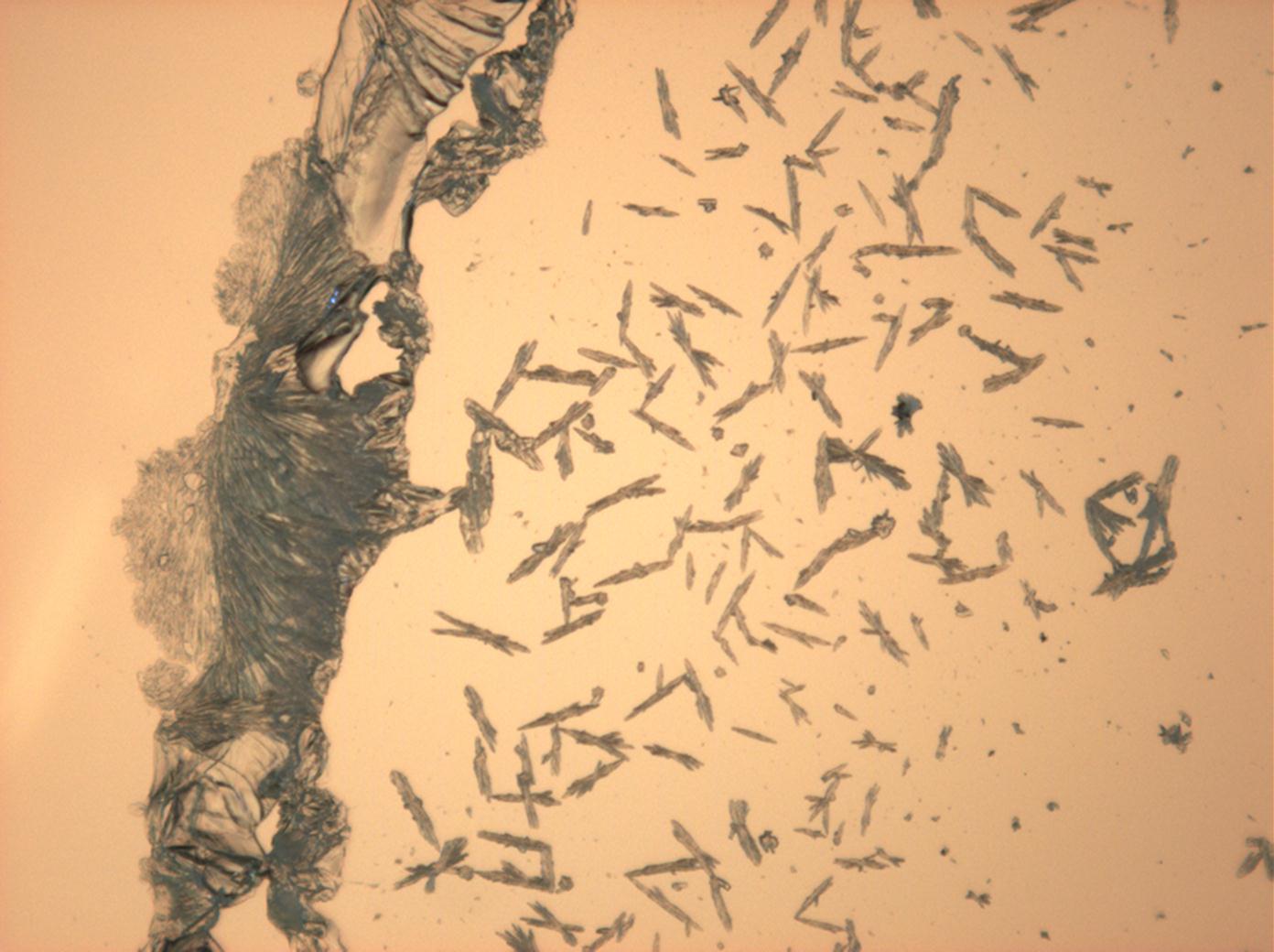
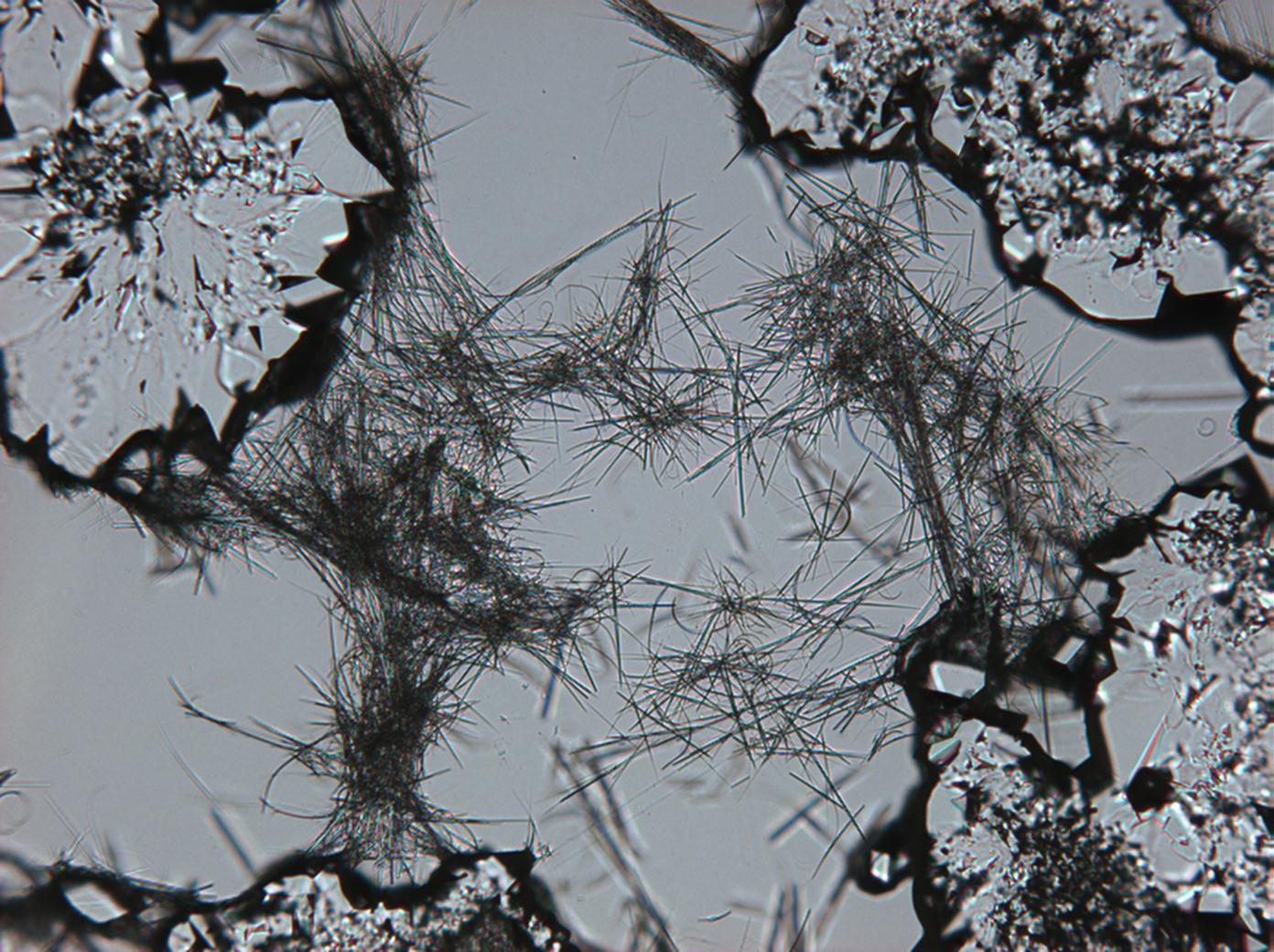
In Abhängigkeit von den vorliegenden Luftfeuchte- und Temperaturbedingungen verändern Kristalle des Rohprobematerials und des rekristallisierten Präparates ihren Kristallwassergehalt. An trockner Luft (mit r.F. < 80% und Raumtemperatur) verliert Mirabilit sein Kristallwasser und geht in Thenardit über. Dieser Vorgang kann mikroskopisch klar nachvollzogen werden, wenn der Prozess der Rekristallisation beobachtet wird. Mirabilit weist charakteristische anormale Interferenzfarbe auf, im Zuge des Wasserverlustes und Entstehen von Thenardit schwächen sich die anormalen Interferenzphänomene zunehmend ab.
Die Zuweisung der Brechungsindizes von Thenardit erfolgt entsprechend der Immersionsmethode. Aufgrund der niedrigen maximalen Doppelbrechung zeigt Thenardit zumeist graue Interferenzfarben. Die Auslöschung ist parallel oder symmetrisch.
Verwechslungsmöglichkeiten:
Generell ist die Unterscheidung einer bestimmten Anzahl von Sulfaten (die unten aufgelistet sind und wozu Thenardit zählt) ohne mikrochemische Bestimmung der Anionen problematisch, da die Brechungsindizes der Salze dicht beieinander liegen und alle Salze eine niedrige Doppelbrechung aufweisen. Hilfreich ist die Verwendung eines Immersionsmittels mit einem nD-Wert von 1,48. Eine Differenzierung innerhalb dieser Gruppe wird damit möglich. Außerdem können die unten genannten Eigenschaften als Abgrenzungskriterien hinzugezogen werden.
Eindeutig bestimmbar wird Thenardit durch die Möglichkeit, nach Auflösung des Probematerials im Zuge der Rekristallisation das Phänomen anormaler Interferezfarben beobachten zu können, sprich Mirabilit in der hohen Hydratstufe zu identifizieren und somit indirekt Thenardit nachzuweisen.
| Salzphase | Unterscheidungsmerkmal |
| Boussingaultit (NH4)2Mg(SO)4 • 6H20 | keine anormalen Interferenzfarben / schiefe Auslöschung |
| Pikromerit K2Mg(SO4)2 • 6H20 | keine anormalen Interferenzfarben / schiefe Auslöschung |
| Bloedit Na2Mg(SO4)2 • 6H20 | alle Indizes >1.48 / keine anormalen Interferenzfarben / schiefe Auslöschung / optisch negativ orientiert. |
| Glaserit K3Na(SO4)2 | alle Indizes >1.48 / keine anormalen Interferenzfarben/schiefe Auslöschung |
| Arcanit K2SO4 | alle Indizes >1.48 / keine anormalen Interferenzfarben |
| Magnesiumformiat Mg(HCO2)2 • 2H2O | vergleichsweise hohe Doppelbrechung / keine anormalen Interfernzfarben / schiefe Auslöschung |
Betrachtung von Mischsystemen:
Mischsystem Na+– Ca2+– SO4 2-: Der Ausfall von Gips erfolgt im Zuge der Rekristallisation entsprechend der geringeren Löslichkeit desselben zuerst. Der charakteristische nadelige Habitus von einzelnen Gipskristallen wie auch von Aggregaten bleibt bestehen. Der Ausfall von Natriumsulfat erfolgt später, das eigentliche Kristallwachstum vollzieht sich merklich schneller. Die Morphologie ist unspezifisch.
Mischsystem Na+– SO4 2-– Cl-: Der Ausfall der beiden Partikelsorten beginnt etwa zeitgleich. Halit mit charakteristischer Morphologie, Natriumsulfat in extrem variierender Gestalt.
Pictures of salt and salt damage[edit]
Am Objekt[edit]
- Thenardit Ausblühungen an Objekten
- Eilsum Gipsausbluehungen.jpg
Thenarditausblühungen in der Ev. Ref. Kirche in Eilsum
Under the polarized microscope[edit]
- Natriumsulfat-Kristalle zwischen zwei Objektträgern kristallisiert
- NNatriumsulfat-Kristalle , kristallisiert aus wässriger Lösung von Realproben
Unter dem Rasterelektronenmikroskop[edit]
Weblinks
[edit]
- ↑ http://webmineral.com/data/Thenardite.shtml viewed on 29/07/2010
- ↑ http://www.mindat.org/min-3935.html viewed on 29/07/2010
Literatur[edit]
[Filter missing]