What are salts?: Difference between revisions
| (5 intermediate revisions by 3 users not shown) | |||
| Line 1: | Line 1: | ||
Authors: [[user:Hschwarz|Hans-Jürgen Schwarz]] | |||
<br>back to [[Fundamentals]] | <br>back to [[Fundamentals]] | ||
== Abstract == | == Abstract == | ||
Salts are compounds made up of cations (e.g., K<sup>+</sup>, Ca<sup>2+</sup>, NH<sub>4</sub><sup>+</sup>) and | Salts are compounds made up of cations (e.g., K<sup>+</sup>, Ca<sup>2+</sup>, NH<sub>4</sub><sup>+</sup>) and anions, such as chloride (Cl<sup>-</sup>), nitrate (NO<sub>3</sub><sup>-</sup>, that are held together by ionic bonds. They are crystalline materials.<br> | ||
== The formation of salts == | == The formation of salts == | ||
| Line 13: | Line 11: | ||
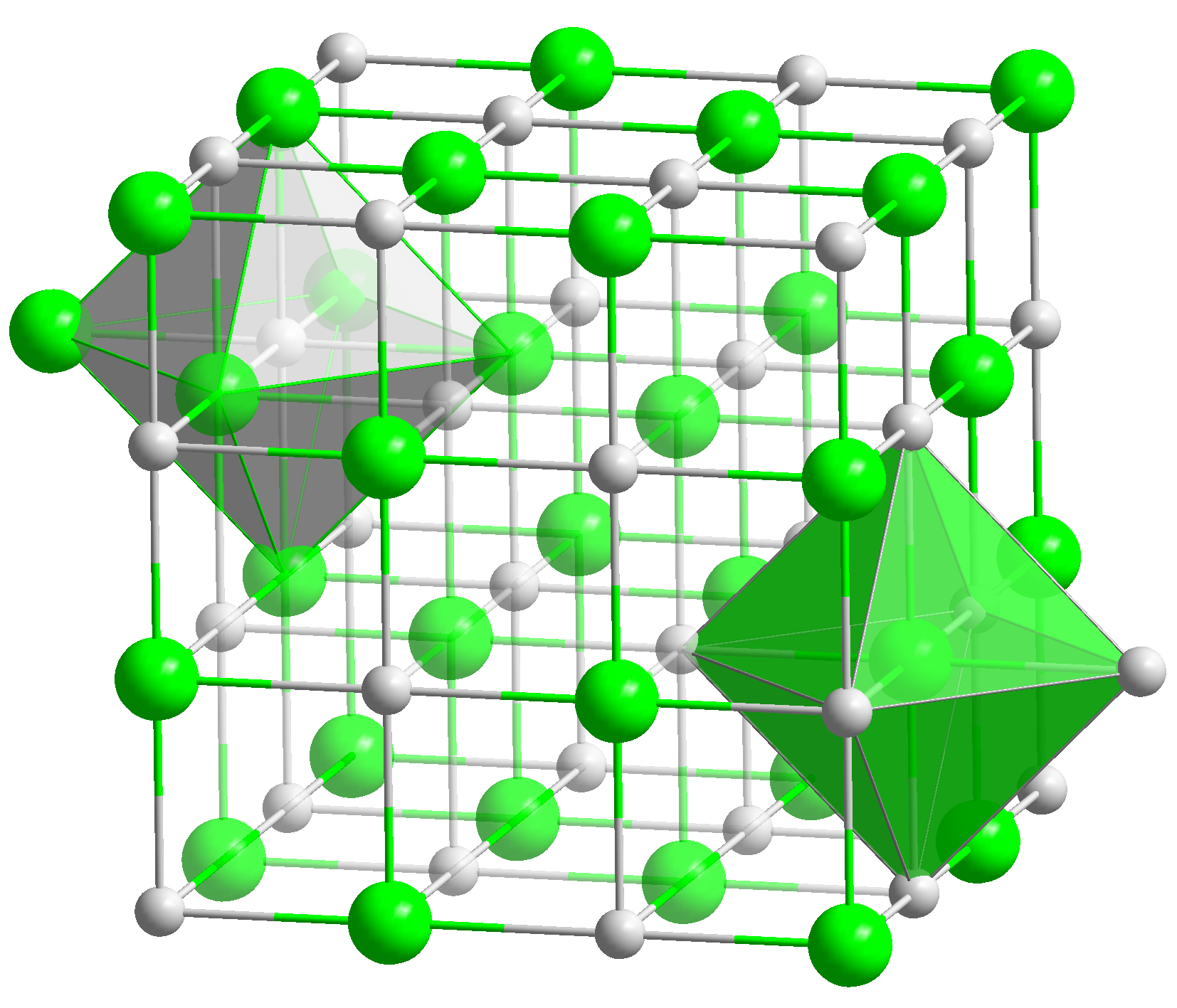
[[Image:NaCl polyhedra.png|thumb|right|200px|Fig. 1:The crystal lattice of NaCl]] | [[Image:NaCl polyhedra.png|thumb|right|200px|Fig. 1:The crystal lattice of NaCl]] | ||
Salts are | Salts, by definition, are the result of a neutralization reaction, i.e., the mixture of an acid with a base.<br> | ||
<br><math>Na^+ + OH^- + H_3O^+ + Cl^- \rightarrow NaCl + 2H_2O</math><br> | <br><math>Na^+ + OH^- + H_3O^+ + Cl^- \rightarrow NaCl + 2H_2O</math><br> | ||
base + acid → salt + water | base + acid → salt + water | ||
| Line 32: | Line 30: | ||
3. <math>H_3O^ + HCO_3^- + 2Na^+ + 2OH^- \rightarrow Na_2CO_3 + 3H_2O </math> | 3. <math>H_3O^ + HCO_3^- + 2Na^+ + 2OH^- \rightarrow Na_2CO_3 + 3H_2O </math> | ||
Another important point is that salts may have different solubilites. While some salts are very soluble, others may be practically insoluble. In general, soluble salts are more "damaging" to inorganic porous materials than the less soluble ones. | |||
| Line 41: | Line 42: | ||
{|cellspacing="0" cellpadding = "10" style="border-style:solid; border-color:black; border-width:1px;" | {|cellspacing="0" cellpadding = "10" style="border-style:solid; border-color:black; border-width:1px;" | ||
|align="center" bgcolor="#ffff99" |Salts consist of cations and anions that are bound together | |align="center" bgcolor="#ffff99" |Salts consist of cations and anions that are bound together by <b style="color:red">ionic bonds</b>. <br> | ||
They are crystalline materials. | They are crystalline materials that may have a wide range of solubilities. | ||
|} | |} | ||
== Composition of salts | == Composition of salts == | ||
The most | The most frequently found [[salts]] consist of these <br> | ||
'''anions''': | '''anions''': | ||
* | *Sulfate SO<sub>4</sub><sup>2-</sup> | ||
*Nitrate NO<sub>3</sub>- | *Nitrate NO<sub>3</sub>- | ||
*Chloride Cl<sup>-</sup> | *Chloride Cl<sup>-</sup> | ||
| Line 76: | Line 77: | ||
*[[solubility]] | *[[solubility]] | ||
*[[deliquescence humidity]] | *[[deliquescence humidity]] | ||
[[Salt properties|Other properties]] of salts, such as density and crystallographic data, are described elsewhere. | |||
==Weblinks== | ==Weblinks== | ||
<references/> | <references/> | ||
[[Category:Fundamentals]] [[Category: | [[Category:Fundamentals]] [[Category:Schwarz,Hans-Jürgen]] [[Category: Approved]] | ||
Latest revision as of 13:16, 14 August 2012
Authors: Hans-Jürgen Schwarz
back to Fundamentals
Abstract[edit]
Salts are compounds made up of cations (e.g., K+, Ca2+, NH4+) and anions, such as chloride (Cl-), nitrate (NO3-, that are held together by ionic bonds. They are crystalline materials.
The formation of salts[edit]
Salts[1] normally consist of positively charged ions, cations, and negatively charged ions, anions, that form a crystal lattice. In addition, some salts may include the water molecule (H2O) in the lattice. there are referred to as hydrated salts.
Salts, by definition, are the result of a neutralization reaction, i.e., the mixture of an acid with a base.
Failed to parse (SVG (MathML can be enabled via browser plugin): Invalid response ("Math extension cannot connect to Restbase.") from server "https://wikimedia.org/api/rest_v1/":): {\displaystyle Na^+ + OH^- + H_3O^+ + Cl^- \rightarrow NaCl + 2H_2O}
base + acid → salt + water
While some salts, such as NaCl are neutral, that is, their solution does not change the normal pH 7 of water, other salts may be alkaline or acidic, depending on the strength of the participating acids and bases:
1. strong acid + strong base → neutral salt + H2O
2. strong acid + weak base → acidic salt + H2O
3. weak acid + strong base → alkaline salt + H2O
e.g.
1. Failed to parse (SVG (MathML can be enabled via browser plugin): Invalid response ("Math extension cannot connect to Restbase.") from server "https://wikimedia.org/api/rest_v1/":): {\displaystyle H_3O^+ + Cl^- + Na^+ + OH^- \rightarrow NaCl + H_2O }
2. Failed to parse (SVG (MathML can be enabled via browser plugin): Invalid response ("Math extension cannot connect to Restbase.") from server "https://wikimedia.org/api/rest_v1/":): {\displaystyle H_3O^+ + Cl^- + NH_3 \rightarrow NH_4Cl + H_2O }
3. Failed to parse (SVG (MathML can be enabled via browser plugin): Invalid response ("Math extension cannot connect to Restbase.") from server "https://wikimedia.org/api/rest_v1/":): {\displaystyle H_3O^ + HCO_3^- + 2Na^+ + 2OH^- \rightarrow Na_2CO_3 + 3H_2O }
Another important point is that salts may have different solubilites. While some salts are very soluble, others may be practically insoluble. In general, soluble salts are more "damaging" to inorganic porous materials than the less soluble ones.
The chemical notation of salts is written like this:
(positive ion (cation))x (negative ion (anion))y • nH2O; e.g. CaCl2 • 6 H2O ,
x,y,n being the number of the ions or water molecules
| Salts consist of cations and anions that are bound together by ionic bonds. They are crystalline materials that may have a wide range of solubilities. |
Composition of salts[edit]
The most frequently found salts consist of these
anions:
- Sulfate SO42-
- Nitrate NO3-
- Chloride Cl-
- Carbonate CO32-
And the cations
- Sodium Na+
- Potassium K+
- Calcium Ca2+
- Magnesium Mg2+
other anions are:
- Acetate CH3COO-
- Formiate HCOO-
- Oxalate C2O42-
- Phosphate PO43-
further cations are:
- Ammonium NH4+
Salt properties[edit]
Salts have certain properties that help to explain their behaviour in solution and their "damaging" effect on objects. The most important are:
Other properties of salts, such as density and crystallographic data, are described elsewhere.