Sodium sulfate phase III
Author: Amelie Stahlbuhk
back to Sulfate
| Sodium sulfate phase III | |
| Mineralogical name | |
| Chemical name | sodium sulfate phase III |
| Trivial name | |
| Chemical formula | Na2SO4 |
| Other forms | Na2SO4•10H2O (Mirabilite) Na2SO4•7H2O (Sodium sulfate heptahydrate) |
| Crystal system | |
| Crystal structure | |
| Deliquescence humidity 20°C | 82.9 % |
| Solubility (g/l) at 20°C | 4.428 mol/kg |
| Density (g/cm³) | |
| Molar volume | |
| Molar weight | 142.04 g/mol |
| Transparency | |
| Cleavage | |
| Crystal habit | |
| Twinning | |
| Phase transition | |
| Chemical behavior | |
| Comments | |
| Crystal Optics | |
| Refractive Indices | |
| Birefringence | |
| Optical Orientation | |
| Pleochroism | |
| Dispersion | |
| Used Literature | |
| [Steiger.etal:2008]Title: Crystallization of sodium sulfate phases in porous materials: The phase diagram Na2SO4–H2O and the generation of stress Author: Steiger, Michael; Asmussen, Sönke 
| |
Abstract[edit]
Sodium sulfate phase III as a metastable and anhydrous phase of sodium sulfate and its properties are presented.
Occurence[edit]
The phase was observed next to thenardite during the evaporation of sodium sulfate solutions above 32.4 °C which is above the transition temperature of mirabilite to thenardite. The amount of metastable phase III increases with increasing temperature [Amirthalingam.etal:1977]Title: Topotaxic phase change in Na2SO4
Author: Amirthalingam V., Karkhanavala M. D., Rao U. R. K. , [Grossi.etal:1997]Title: Acoustic emmission monitoring to study sodium sulphate crystallization in monumental porous carbonate stones
, [Grossi.etal:1997]Title: Acoustic emmission monitoring to study sodium sulphate crystallization in monumental porous carbonate stones
Author: Grossi, C.M.; Esbert, R.M.; Suarez del Rio, L.M.; Montoto, M.; Laurenzi-Tabasso, M. .
.
In addition the phase was detected during evaporation tests with sodium sulfate solutions at room temperature and low relative humidity [Linnow.etal:2006]Title: Investigation of Sodium Sulfate Phase Transitions in a Porous Material Using Humidity- and Temperature-Controlled X-ray Diffraction
Author: Linnow, Kirsten; Zeunert, Anke; Steiger, Michael , [Xu.etal:1999]Title: In-situ Raman observations of phase transformation of Na2SO4 during the hydration/dehydration cycles on single levitated microparticle.
, [Xu.etal:1999]Title: In-situ Raman observations of phase transformation of Na2SO4 during the hydration/dehydration cycles on single levitated microparticle.
Author: Xu B., Schweiger G. , [Rodriguez-Navarro.etal:1999]Title: How does sodium sulphate crystallize?
, [Rodriguez-Navarro.etal:1999]Title: How does sodium sulphate crystallize?
Author: Rodriguez-Navarro, Carlos; Doehne, Eric .
.
Solubility[edit]
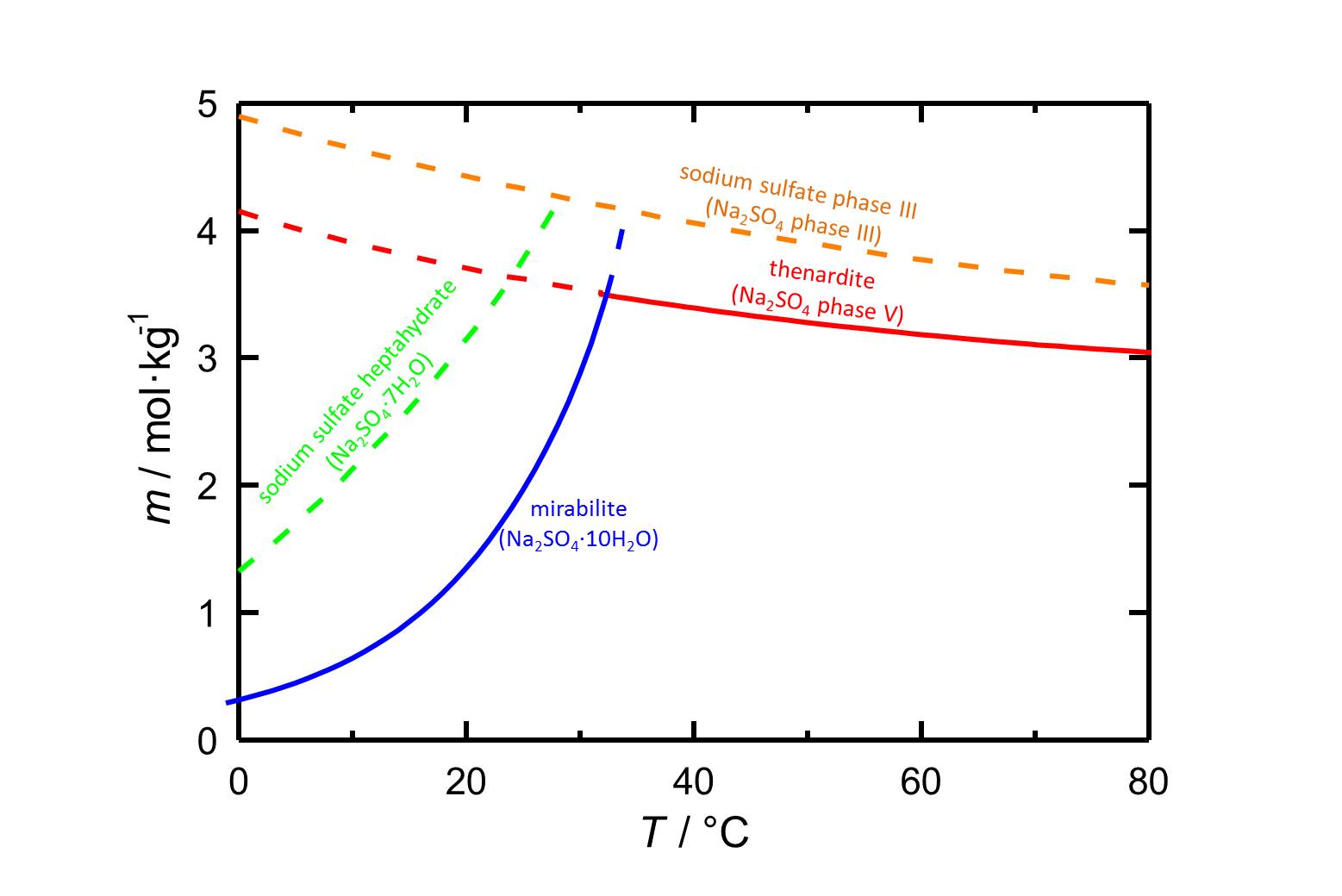
Author: Steiger, Michael; Asmussen, Sönke

With a solubility of 4.4 mol/kg at 20 °C [Steiger.etal:2008]Title: Crystallization of sodium sulfate phases in porous materials: The phase diagram Na2SO4–H2O and the generation of stress
Author: Steiger, Michael; Asmussen, Sönke phase III has got a higher solubility than the other phases of the system Na2SO4-H2O (see also sodium sulfate).
phase III has got a higher solubility than the other phases of the system Na2SO4-H2O (see also sodium sulfate).
Hygroscopicity[edit]
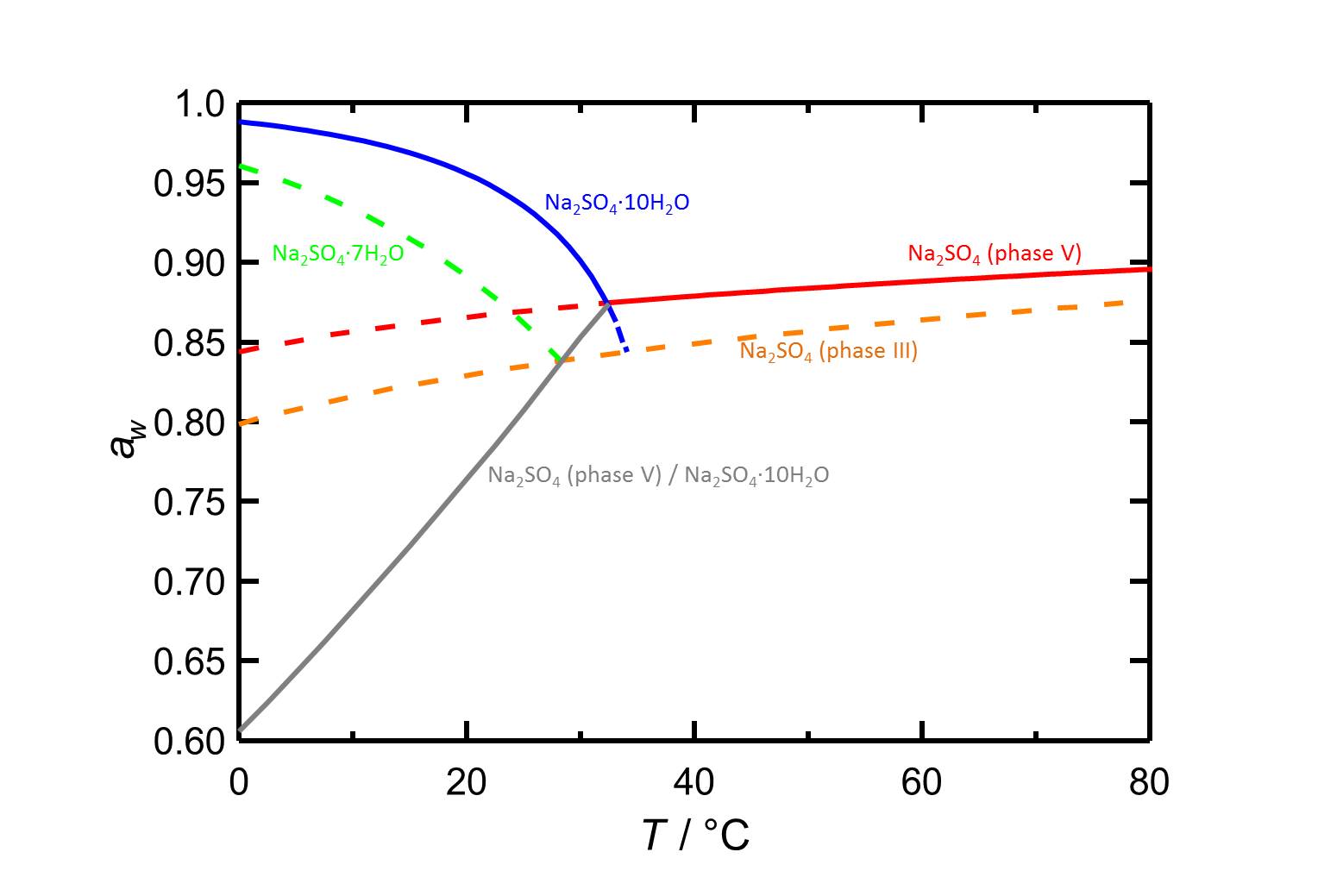
Author: Steiger, Michael; Asmussen, Sönke

The deliquescence humidity of phase III at 20 °C is 82.9 % and it slowly increases with increasing temperature.
| 0°C | 10°C | 20°C | 30°C | 40°C | 50°C |
| 79.9%r.h. | 81.6%r.h. | 82.9%r.h. | 84.0%r.h. | 84.9%r.h. | 85.7%r.h. |
Weblinks
[edit]
Literatur[edit]
| [Amirthalingam.etal:1977] | Amirthalingam V., Karkhanavala M. D., Rao U. R. K. (1977): Topotaxic phase change in Na2SO4. In: Acta Cryst., (), 522 |  |
| [Grossi.etal:1997] | Grossi, C.M.; Esbert, R.M.; Suarez del Rio, L.M.; Montoto, M.; Laurenzi-Tabasso, M. (1997): Acoustic emmission monitoring to study sodium sulphate crystallization in monumental porous carbonate stones. In: Studies in Conservation, (2), 115-125 |  |
| [Linnow.etal:2006] | Linnow, Kirsten; Zeunert, Anke; Steiger, Michael (2006): Investigation of Sodium Sulfate Phase Transitions in a Porous Material Using Humidity- and Temperature-Controlled X-ray Diffraction. In: Analytical Chemistry, 78 (13), 4683-4689, 10.1021/ac0603936 |  |
| [Rodriguez-Navarro.etal:1999] | Rodriguez-Navarro, Carlos; Doehne, Eric (2000): How does sodium sulphate crystallize?. In: Cement and Concrete Research, 30 (), 1527-1534, Url, 10.1016/S0008-8846(00)00381-1 |  |
| [Steiger.etal:2008] | Steiger, Michael; Asmussen, Sönke (2008): Crystallization of sodium sulfate phases in porous materials: The phase diagram Na2SO4–H2O and the generation of stress. In: Geochimica et Cosmochimica Acta, 72 (17), 4291-4306, Url |  |
| [Xu.etal:1999] | Xu B., Schweiger G. (1999): In-situ Raman observations of phase transformation of Na2SO4 during the hydration/dehydration cycles on single levitated microparticle.. In: J. Aerosol. Sci., (), 379-380 |  |