Calcium chloride: Difference between revisions
Jump to navigation
Jump to search
No edit summary |
|||
| Line 18: | Line 18: | ||
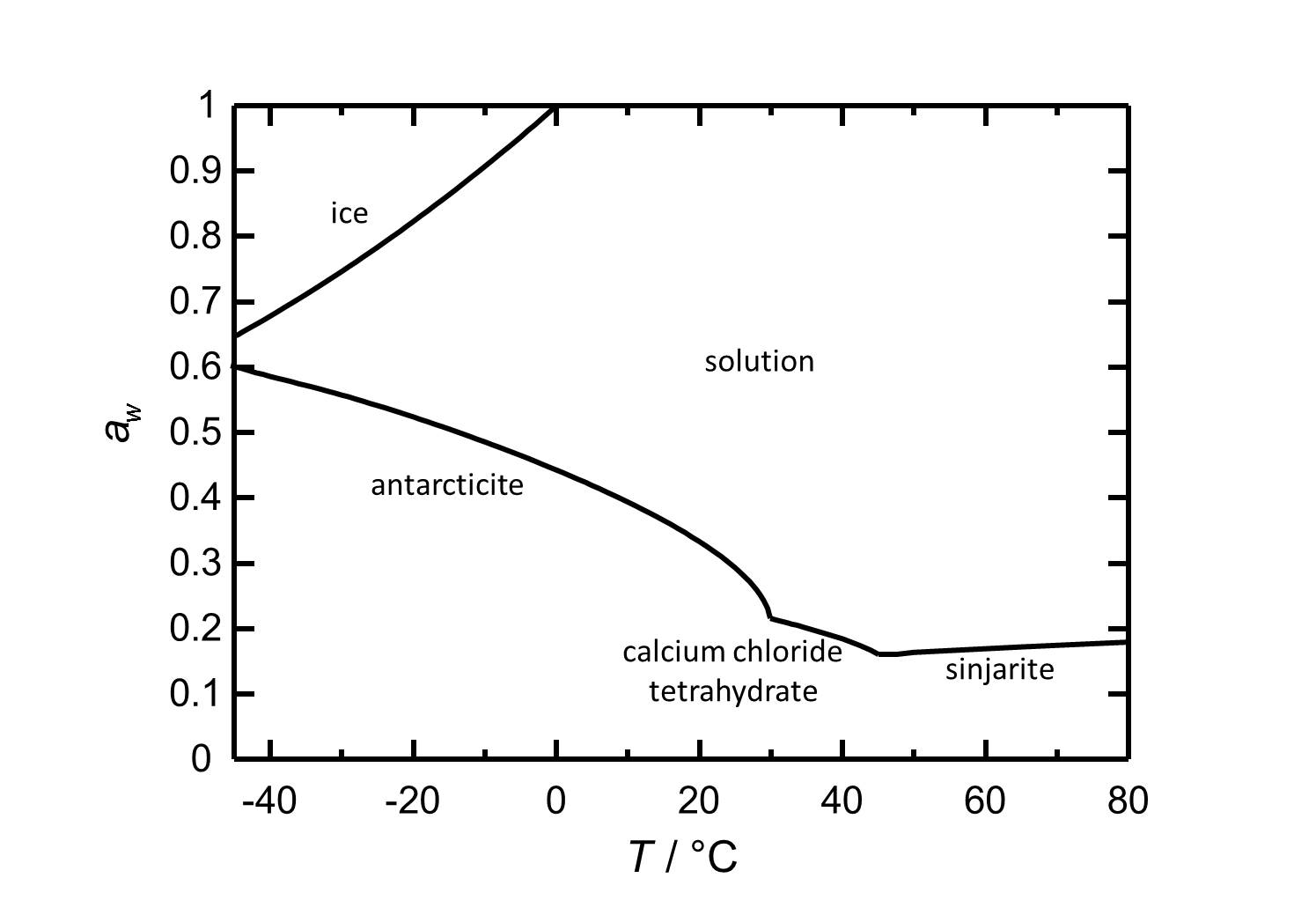
==Hygroscopicity== | ==Hygroscopicity== | ||
[[Image:D CaCl2e.jpg|thumb|left|300px|Figure 2: Deliquescence behaviour of calcium chloride in the temperature range from -45 to 80 °C. The water activity a<sub>w</sub> is plotted against the temperature.]] | |||
==References== | ==References== | ||
Revision as of 14:27, 24 February 2015
Author: Amelie Stahlbuhk
back to Chloride
| This article will be released soon. |
Abstract[edit]
The different hydrate stages of calcium chloride are presented, as well as their behavior regarding solubility and hygroscopicity.
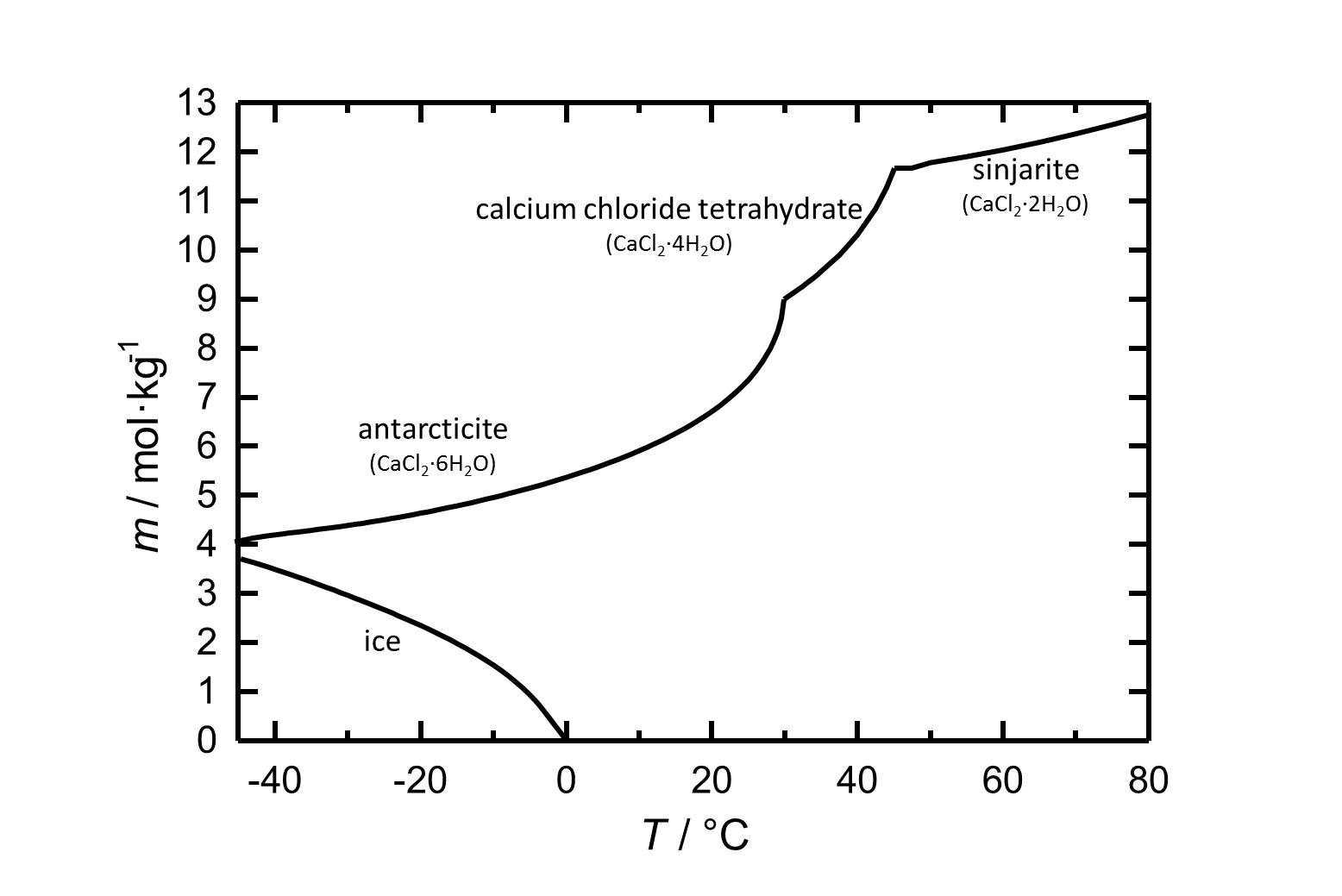
Hydrate stages[edit]
Sinjarite: CaCl2•2H2O
Calcium chloride tetrahydrate: CaCl2•4H2O
Antarcticite: CaCl2•6H2O