Calcium chloride: Difference between revisions
Jump to navigation
Jump to search
No edit summary |
No edit summary |
||
| Line 15: | Line 15: | ||
==Solubility== | ==Solubility== | ||
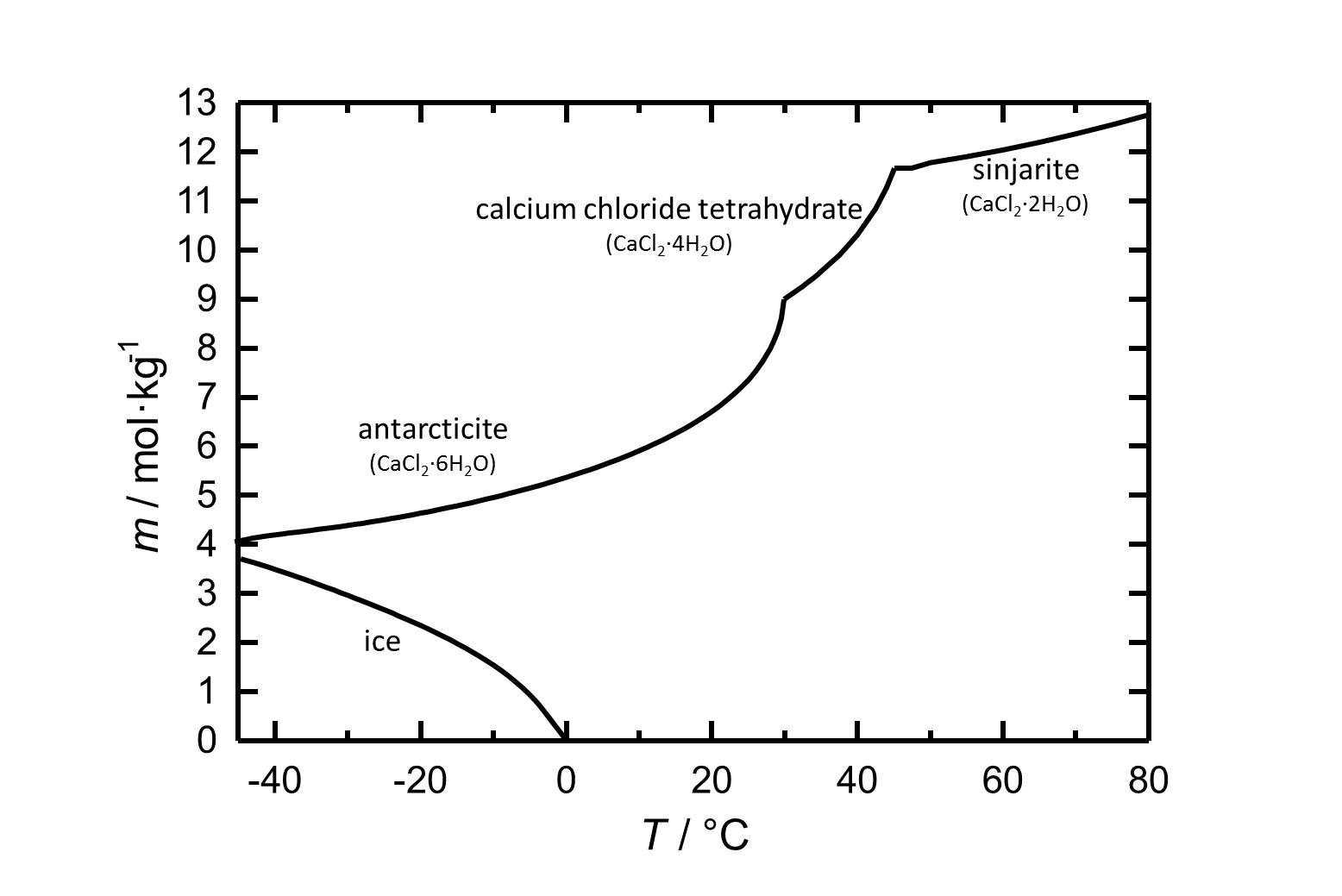
[[file:L CaCl2 d.jpg|thumb|left| | [[file:L CaCl2 d.jpg|thumb|left|300px|Figure 1: Solubility of calcium chloride in water. The molality ''m'' [n(CaCl<sub>2</sub>•xH<sub>2</sub>O)•kg(H<sub>2</sub>O)<sup>-1</sup>] is plotted against the temperature.]] | ||
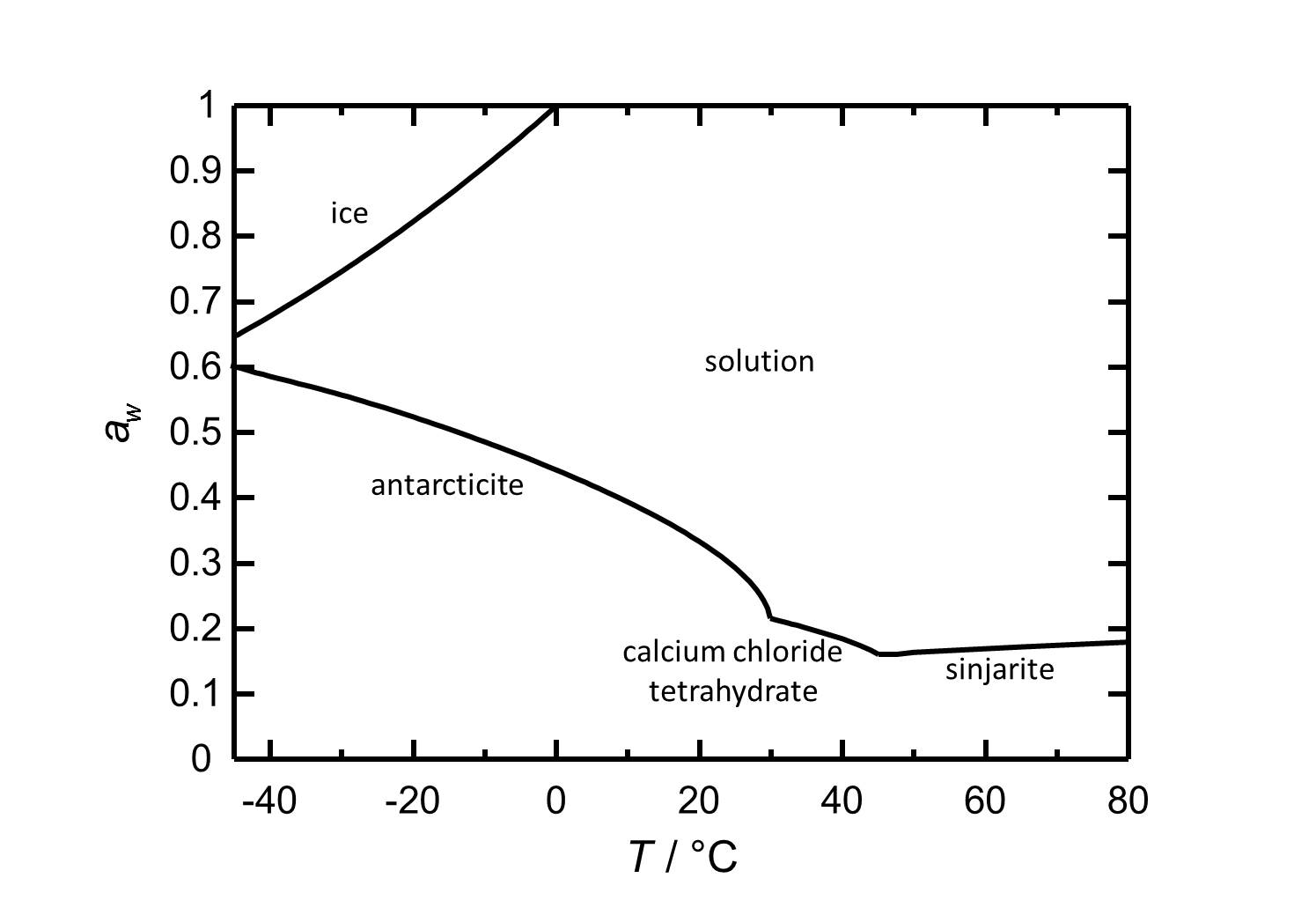
==Hygroscopicity== | ==Hygroscopicity== | ||
Revision as of 21:16, 24 February 2015
Author: Amelie Stahlbuhk
back to Chloride
| This article will be released soon. |
Abstract[edit]
The different hydrate stages of calcium chloride are presented, as well as their behavior regarding solubility and hygroscopicity.
Hydrate stages[edit]
Sinjarite: CaCl2•2H2O
Calcium chloride tetrahydrate: CaCl2•4H2O
Antarcticite: CaCl2•6H2O