Mirabilite: Difference between revisions
No edit summary |
|||
| Line 8: | Line 8: | ||
|mineralogical_Name = Mirabilite | |mineralogical_Name = Mirabilite | ||
|chemical_Name = Sodium sulfate decahydrate | |chemical_Name = Sodium sulfate decahydrate | ||
|Trivial_Name = Glauber salt, Reussin, | |Trivial_Name = Glauber salt, Reussin, Soda Sulfate | ||
|chemical_Formula = Na<sub>2</sub>SO<sub>4</sub>•10H<sub>2</sub>O | |chemical_Formula = Na<sub>2</sub>SO<sub>4</sub>•10H<sub>2</sub>O | ||
|Hydratforms = Sodium sulphate heptahydrate Na<sub>2</sub>SO<sub>4</sub>•7H<sub>2</sub>O | |Hydratforms = Sodium sulphate heptahydrate Na<sub>2</sub>SO<sub>4</sub>•7H<sub>2</sub>O | ||
Revision as of 20:46, 7 December 2013
Authors: Hans-Jürgen Schwarz, Nils Mainusch
English version by Christa Gerdwilker
back to back to Sulfate
| Mirabilite[1][2] | |
| |
| Mineralogical name | Mirabilite |
| Chemical name | Sodium sulfate decahydrate |
| Trivial name | Glauber salt, Reussin, Soda Sulfate |
| Chemical formula | Na2SO4•10H2O |
| Other forms | Sodium sulphate heptahydrate Na2SO4•7H2O |
| Crystal system | monoclinic |
| Crystal structure | |
| Deliquescence humidity 20°C | 93.6% (20°C), 90% (23°C), 87% (25°C) |
| Solubility (g/l) at 20°C | 900 g/l |
| Density (g/cm³) | 1.464 g/cm³ |
| Molar volume | 219.8 cm3/mol |
| Molar weight | 322.21 g/mol |
| Transparency | transparent to opaque |
| Cleavage | perfect |
| Crystal habit | |
| Twinning | |
| Phase transition | |
| Chemical behavior | |
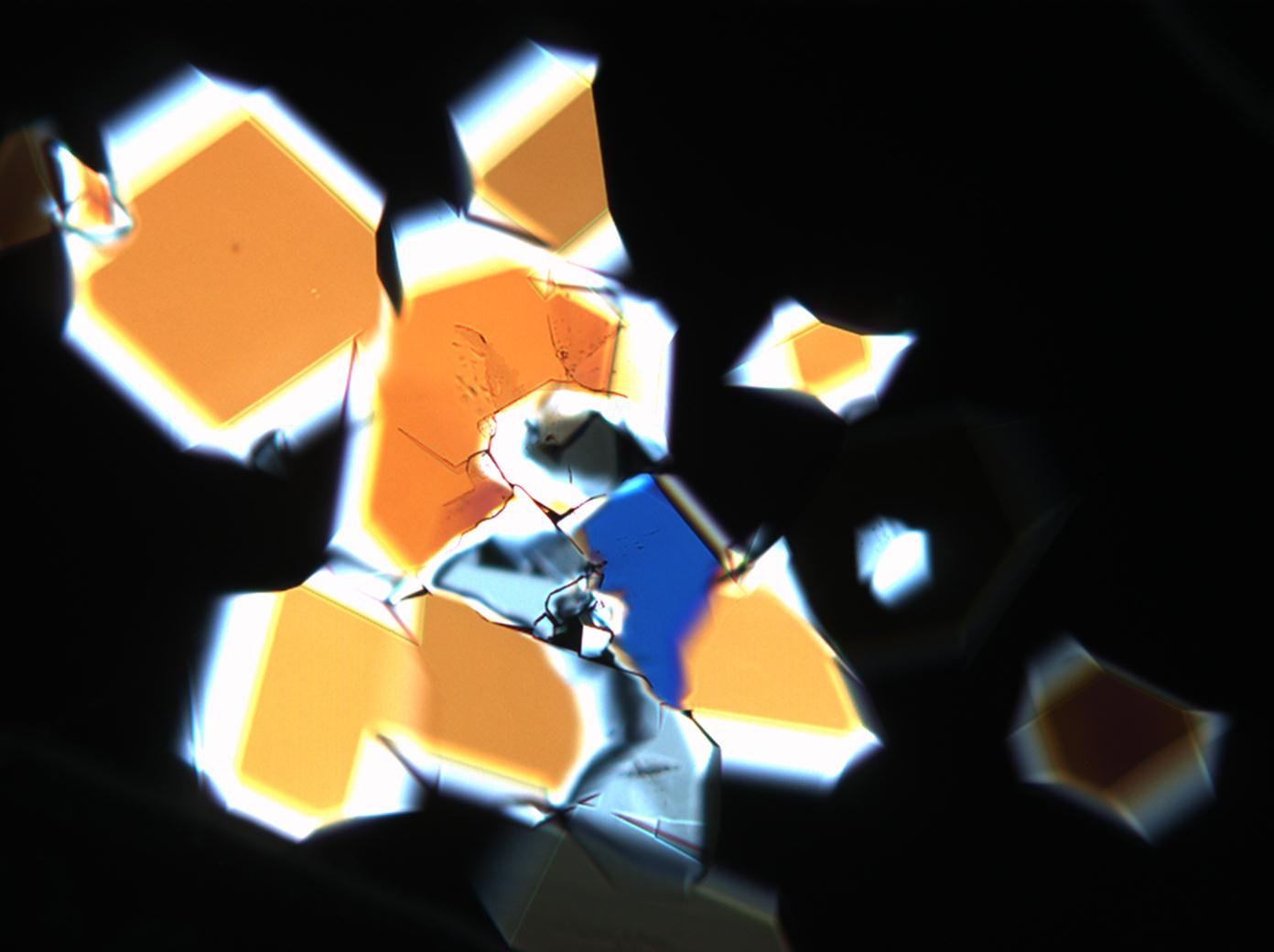
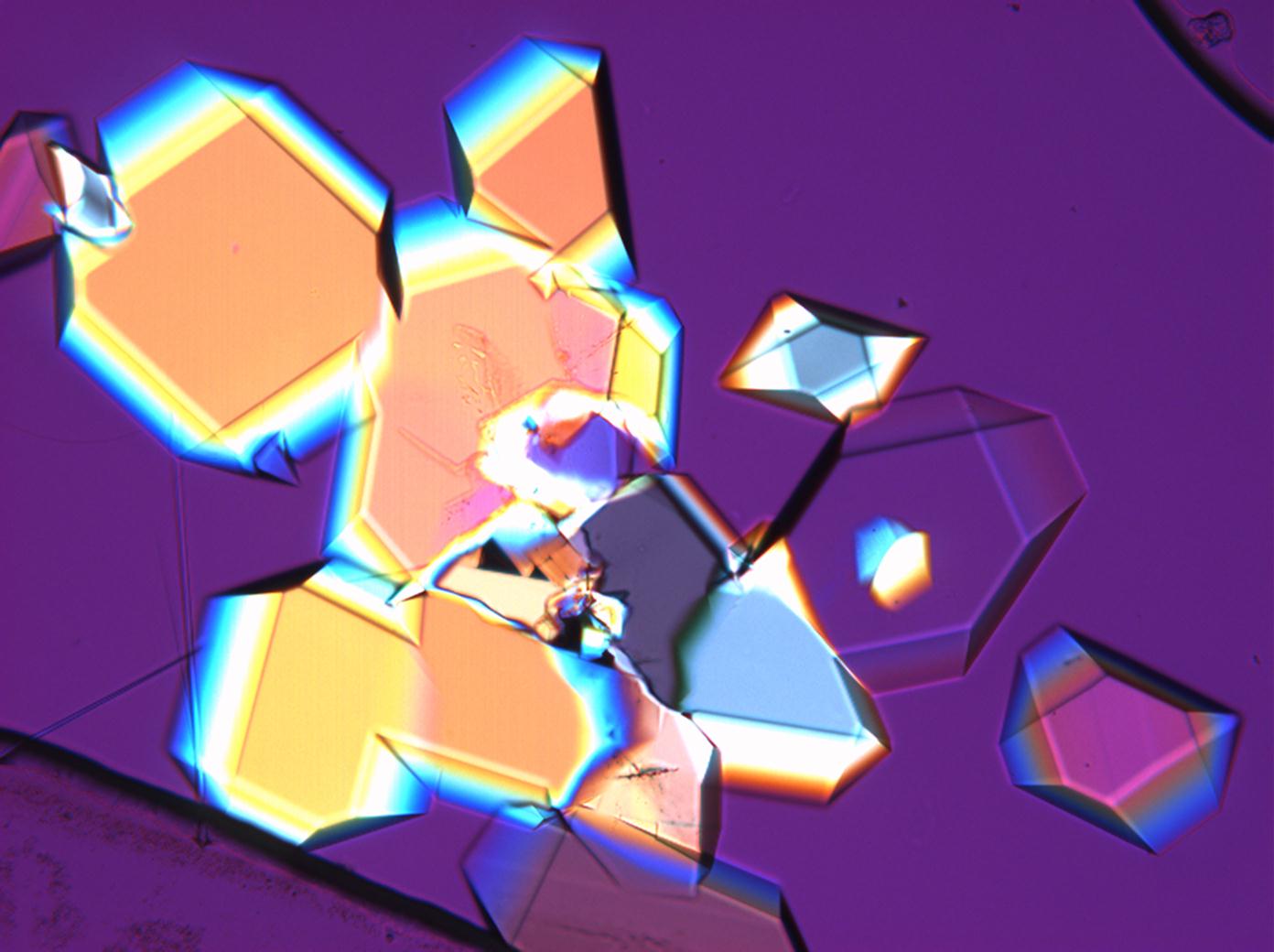
| Comments | soluble in water and glycerin, not soluble in pure alcohol easily loses some water, converts to thenardite at 32°C abnormal blue or brown interference colors |
| Crystal Optics | |
| Refractive Indices | nx = 1.395 ny = 1.396-1.410 nz = 1.398-1.419 |
| Birefringence | Δ = 0.04-0.023 |
| Optical Orientation | negative |
| Pleochroism | |
| Dispersion | |
| Used Literature | |
| {{{Literature}}} | |
Solubility properties[edit]
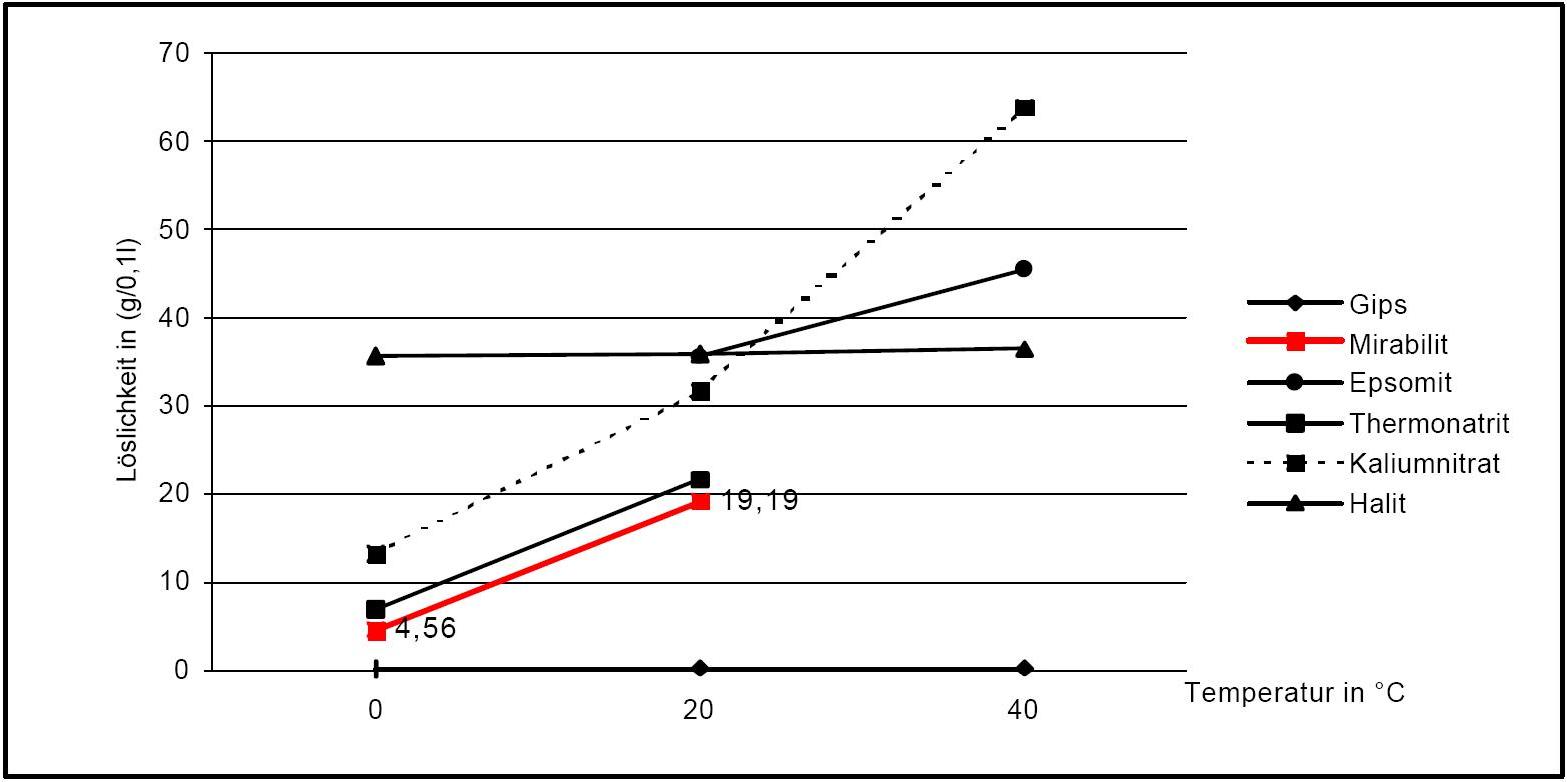
Author: Stark, Jochen; Stürmer, Sylvia
 ].
].
see Sodium sulfate
Hygroscopicity[edit]
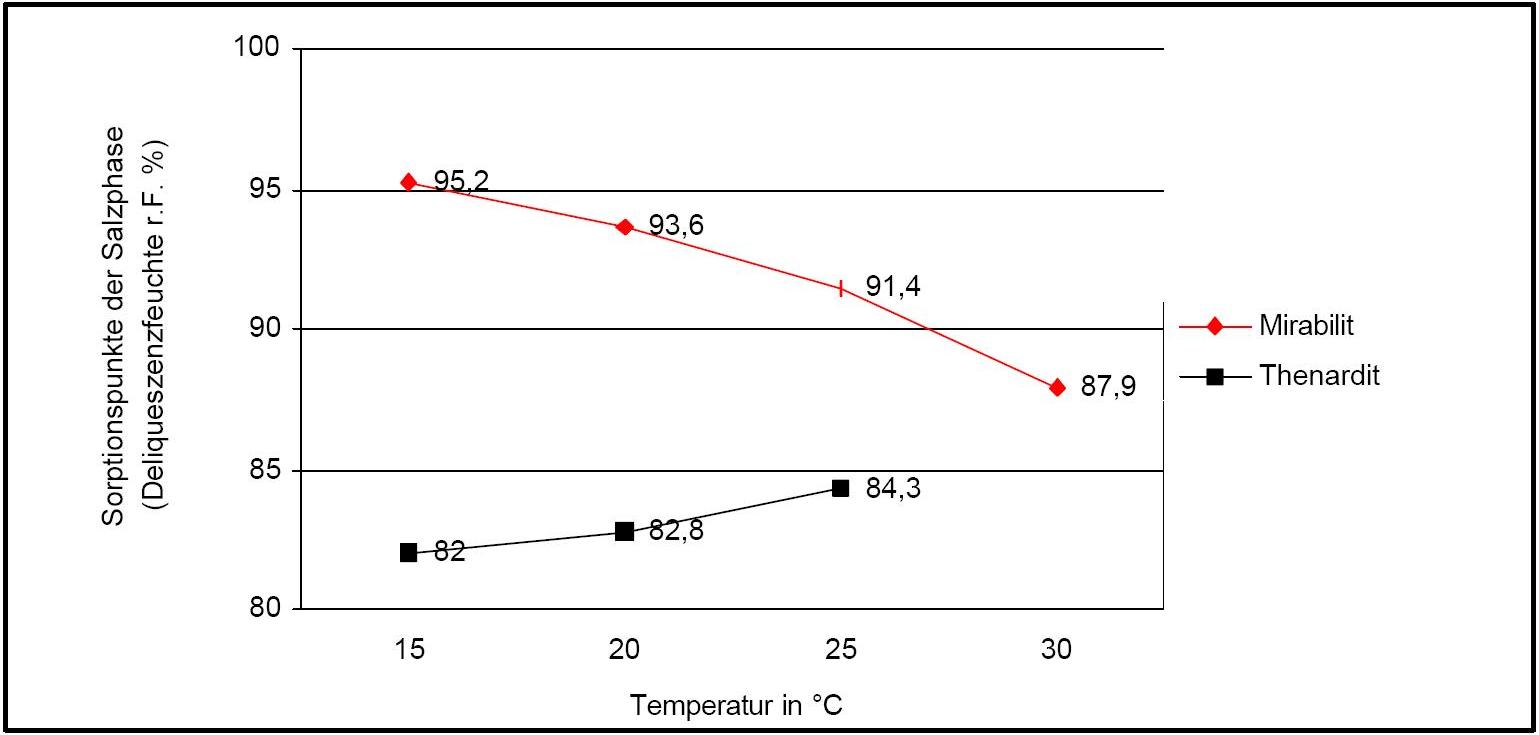
Author: Arnold, Andreas; Zehnder, Konrad

Figure 2 illustrates the influence of temperature on the deliquescence points of thenardite and mirabilite. Note the opposing curves of the graphs.
The presence of other ions (in salt mixtures) can significantly alter the equilibrium humidity parameters, i.e. the temperature and humidity conditions which initiate phase changes. Table 1 lists the experimentally determined equilibrium humidity of different salt mixtures. This shows that the equilibrium humidity of pure mirabilite is higher than that of the other salts.
| MgSO4 | Ca(NO3)2 | KNO3 | |
| Na2SO4 • 10H2O | 87(21°C) | 74 (21°C) | 81(21°C) |
Hygroscopicity
To assess the hygroscopicity of sodium sulfates, the table below compares the moisture sorption of pure sodium sulphate and sodium sulphate-halite mixture at different relative humidity (RH) levels.
| storage relative RH | 87% RH. | 81% RH | 79% RH |
| Na2SO4 | 79 | 0 | 0 |
| Na2SO4+NaCl (1:1 molar mixture) |
157 | 32 | 15 |
Crystallization pressure[edit]
The crystallization of mirabilite from aqueous solution results in a crystallization pressure of 7.2-8.3 N/mm2.
Hydration behavior[edit]
see Sodium sulfate
Analytical evidence[edit]
Microscopy
[edit]
Laboratory analysis:
Observations of the solubility behavior through the microscope allow the verification of sodium sulfate’s good water solubility and insolubility in ethanol. Thenardite and mirabilite do not have morphological characteristics to aid their identification during simple recrystallization experiments. Instead, a broad range of different forms could be observed.
Refractive indices: nx = 1,395; ny =1.396-1.410; nz =1.398-1.419
Birefringence: Δ = 0.04-0,023
Crystal classe: monocline
Under the polarizing microscope:
The water of crystallization content of the sample and re-crystallized material depends on the ambivalent RH and temperature levels. In dry air (with 80% RH at room temperature) mirabilite loses its water of crystallization content and changes to thenardite. This process can be observed during recrystallization under the microscope. Mirabilite has characteristic abnormal interference colours which weaken during water loss and the formation of thenardite.
Differentiation from different salts:
Generally, the differentiation of certain sulfates (listed below and including thenardite) without the microchemical determination of the anions is problematic as their refractive indices are close to each other and all salts display a slight double refraction. The use of an immersion material with a nD-value of 1.48 is helpful and allows the differentiation of salts within this group. Additionally, the properties listed below can also be taken into consideration. Thenardite can be determined indirectly through the observation of mirabilite during the high hydration stages of salt recrystallization.
| Salt phase | Characteristics |
| Boussingaultite (NH4)2Mg(SO)4 • 6H20 | no abnormal interference colors/oblique extinction |
| Picromerite K2Mg(SO4)2 • 6H20 | no abnormal interference colors/oblique extinction |
| Bloedite Na2Mg(SO4)2 • 6H20 | all indices >1.48 / no abnormal interference colors/oblique extinction / negative optical orientation |
| Glaserite K3Na(SO4)2 | all indices >1.48 / no abnormal interference colors/oblique extinction |
| Arcanite K2SO4 | all indices >1.48 / no abnormal interference colors |
| Dashkovaite Mg(HCO2)2 • 2H2O | relatively high birefringence/ no abnormal interference colors/oblique extinction |
Mixtures:
Mixed systems Na+– Ca2+– SO4 2-: Due to its poorer solubility, gypsum precipitates first during re-crystallization. The characteristic needle shapes of the individual gypsum crystals as well as those of the aggregates remain. The precipitation of sodium sulfate occurs later, the crystal growth is noticeably faster, the morphology is non-specific.
Mixed system Na+– SO4 2-– Cl-: The precipitation of both types of particles begins approx. simultaneously. Halite has a characteristic morphology whereas sodium sulfate occurs in extremely varying forms.
Under the polarizing microscope[edit]
- Sodium sulfate crystallization between two glass slides
Weblinks
[edit]
- ↑ http://webmineral.com/data/Mirabilite.shtml accessed 29/07/2010
- ↑ http://www.mindat.org/min-2725.html accessed 29/07/2010
Literatur[edit]
| [Dana:1951] | Dana E.S. (eds.) Dana J.D. (1951): Dana's System of Mineralogy, 7, Wiley & Sons |  |
| [Robie.etal:1978] | Robie R.A., Hemingway B.S.; Fisher J.A. (1978): Thermodynamic properties of minerals and related substances at 298.15 K and 1 bar pressure and higher temperatures. In: U.S. Geol. Surv. Bull, 1452 () |  |
| [Steiger.etal:2008] | Steiger, Michael; Asmussen, Sönke (2008): Crystallization of sodium sulfate phases in porous materials: The phase diagram Na2SO4–H2O and the generation of stress. In: Geochimica et Cosmochimica Acta, 72 (17), 4291-4306, Url |  |
| [Vogt.etal:1993] | Vogt, R.; Goretzki, Lothar (1993): Der Einfluss hygroskopischer Salze auf die Gleichgewichtsfeuchte und Trocknung anorganischer Baustoffe, unveröffentlichter Bericht. |  |