Gypsum: Difference between revisions
No edit summary |
No edit summary |
||
| Line 4: | Line 4: | ||
|mineralogical_Name = Gypsum | |mineralogical_Name = Gypsum | ||
|chemical_Name = Calcium sulfate dihydrate | |chemical_Name = Calcium sulfate dihydrate | ||
|Trivial_Name = | |Trivial_Name = Alabaster, Satin Spar, Selenite | ||
|chemical_Formula = Ca[SO<sub>4</sub>]•2H<sub>2</sub>O | |chemical_Formula = Ca[SO<sub>4</sub>]•2H<sub>2</sub>O | ||
|Hydratforms = Anhydrite (CaSO<sub>4</sub>)<br>Hemihydrate (CaSO<sub>4</sub>•0.5H<sub>2</sub>O) | |Hydratforms = Anhydrite (CaSO<sub>4</sub>)<br>Hemihydrate (CaSO<sub>4</sub>•0.5H<sub>2</sub>O) | ||
| Line 33: | Line 33: | ||
= | =Calcium sulfate and gypsum = | ||
== Abstract == | == Abstract == | ||
The article discusses the system CaSO<sub>4</sub>-H<sub>2</sub>O, in referecne to gypsum. Gypsum is one of the most important salts with regard to the damage of building materials and, particularly, wall-paintings. Objects exposed to exterior conditions where air pollution is present are the most prone to damage from gypsum. The appearance and mechanism of the damage, as well as the examination methods are described. Images, microphotographs and examples from practical experiences illustrate the subject.<br> | |||
<br> | <br> | ||
== Introduction == | == Introduction == | ||
Gypsum is one of the most common salts causing the deterioration of | Gypsum is one of the most common salts causing the deterioration of inorganic porous building materials. It is present on most all exterior exposed surfaces and even in interior environments, under different shapes and induced deterioration patterns. <br> | ||
<br> | <br> | ||
<br> | <br> | ||
== | == Gypsum, one of the most prevalent minerals, forms by precipitation from aqueous solution at temperatures under approximately 40°C. At increased temperatures (> 60°C) of a solution the formation of anhydrite takes place directly. Calcium sulfate and calcium sulfate dihydrate are often present in rocks. Hemihydrate does not occur naturally.<br> | ||
Gypsum occurs naturally in salt deposits and deserts, where the "desert rose" crystals form in combination with quartz inclusions. In salt deposits gypsum and anhydrite sometimes form a caprock, i.e., a massive layer of material covering a natural salt deposit. | |||
Gypsum occurs naturally in salt deposits and | Synthetic gypsum is produced in coal-fired power plants, as a by-product of flue-gas desulfurization. | ||
Synthetic gypsum is produced in coal-fired power plants, as a by-product of | |||
== Origin and formation of gypsum on monuments == | == Origin and formation of gypsum on monuments == | ||
On monuments made from porous inorganic building materials, particularly in urban environment where air pollutants are present, in particular sulfur oxides (SO<sub>x</sub>) that convert to sulfuric acid in the presence of moisture, the reaction of these gases with any calcium carbonate present in limestone, mortars, renders, results in the formation of gypsum. The reaction taking place can be simplified as follows: | |||
CaCO<sub>3</sub> + H<sub>2</sub>SO<sub>4</sub> → CaSO<sub>4</sub> + H<sub>2</sub>O + CO<sub>2</sub> | CaCO<sub>3</sub> + H<sub>2</sub>SO<sub>4</sub> → CaSO<sub>4</sub> + H<sub>2</sub>O + CO<sub>2</sub> | ||
It is to be remembered that gypsum can be a major component of some mortars, plasters and even some building stones (e.g., selenite, used for the base of the Garrisenda Tower in Bologna, Italy) thus being an integral part of the monuments fabric. | |||
<br> | <br> | ||
Revision as of 14:11, 30 November 2013
| Gypsum[1][2] | |

| |
| Mineralogical name | Gypsum |
| Chemical name | Calcium sulfate dihydrate |
| Trivial name | Alabaster, Satin Spar, Selenite |
| Chemical formula | Ca[SO4]•2H2O |
| Other forms | Anhydrite (CaSO4) Hemihydrate (CaSO4•0.5H2O) |
| Crystal system | monoclinic |
| Crystal structure | |
| Deliquescence humidity 20°C | > 99% RH at 20°C |
| Solubility (g/l) at 20°C | 2.14 g/l |
| Density (g/cm³) | 2.2-2.4 g/cm³ |
| Molar volume | 74.69 cm3/mol |
| Molar weight | 172.17g /mol |
| Transparency | transparent to opaque |
| Cleavage | perfect, clearly visible formation of fibre |
| Crystal habit | flat, prismatic, needle- like crystal, granular, massive aggregate |
| Twinning | very common |
| Phase transition | |
| Chemical behavior | hardly soluble in water |
| Comments | |
| Crystal Optics | |
| Refractive Indices | α = 1.519-1.521 β = 1.522-1.523 γ = 1.529-1.530 |
| Birefringence | Δ = 0.010 |
| Optical Orientation | biaxial positive |
| Pleochroism | colorless |
| Dispersion | 58° |
| Used Literature | |
| {{{Literature}}} | |
Authors: Hans-Jürgen Schwarz , Nils Mainusch, Tim Müller
English Translation by Sandra Leithäuser
back to Sulfate
Calcium sulfate and gypsum[edit]
Abstract[edit]
The article discusses the system CaSO4-H2O, in referecne to gypsum. Gypsum is one of the most important salts with regard to the damage of building materials and, particularly, wall-paintings. Objects exposed to exterior conditions where air pollution is present are the most prone to damage from gypsum. The appearance and mechanism of the damage, as well as the examination methods are described. Images, microphotographs and examples from practical experiences illustrate the subject.
Introduction[edit]
Gypsum is one of the most common salts causing the deterioration of inorganic porous building materials. It is present on most all exterior exposed surfaces and even in interior environments, under different shapes and induced deterioration patterns.
== Gypsum, one of the most prevalent minerals, forms by precipitation from aqueous solution at temperatures under approximately 40°C. At increased temperatures (> 60°C) of a solution the formation of anhydrite takes place directly. Calcium sulfate and calcium sulfate dihydrate are often present in rocks. Hemihydrate does not occur naturally.
Gypsum occurs naturally in salt deposits and deserts, where the "desert rose" crystals form in combination with quartz inclusions. In salt deposits gypsum and anhydrite sometimes form a caprock, i.e., a massive layer of material covering a natural salt deposit. Synthetic gypsum is produced in coal-fired power plants, as a by-product of flue-gas desulfurization.
Origin and formation of gypsum on monuments[edit]
On monuments made from porous inorganic building materials, particularly in urban environment where air pollutants are present, in particular sulfur oxides (SOx) that convert to sulfuric acid in the presence of moisture, the reaction of these gases with any calcium carbonate present in limestone, mortars, renders, results in the formation of gypsum. The reaction taking place can be simplified as follows:
CaCO3 + H2SO4 → CaSO4 + H2O + CO2
It is to be remembered that gypsum can be a major component of some mortars, plasters and even some building stones (e.g., selenite, used for the base of the Garrisenda Tower in Bologna, Italy) thus being an integral part of the monuments fabric.
Damage potential and weathering activity[edit]
Solubility properties[edit]
Gypsum belongs to the group of salts with a low solubility in aqueous solution and therefore it can be described as less mobile than others. However, the influence of ions from other sources is comparatively high. For example, the presence of halite can increase the solubility of gypsum in dependence of the concentration ratio, up to the factor of four.
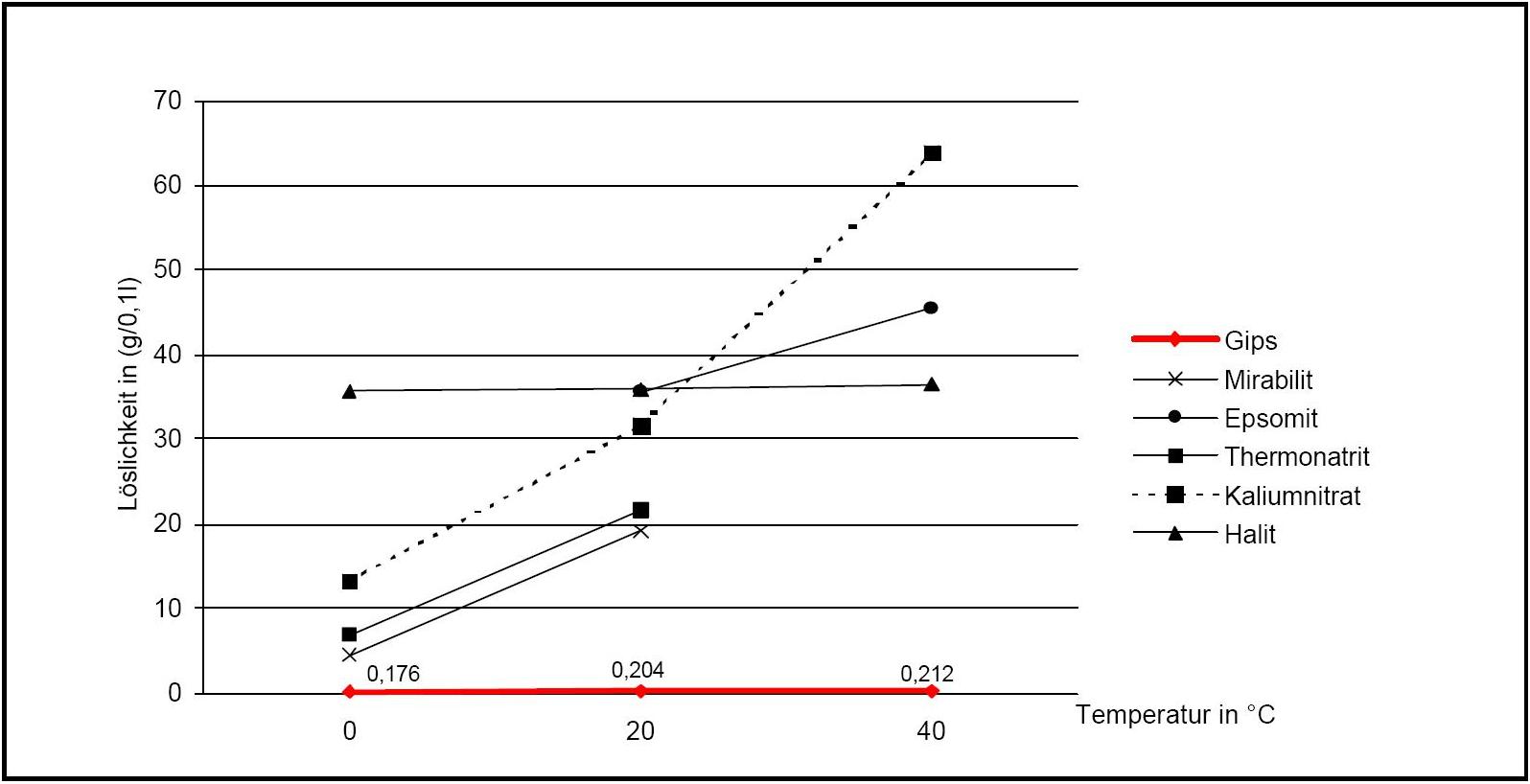
Author: Stark, Jochen; Stürmer, Sylvia
 )
)
Hydration behavior[edit]
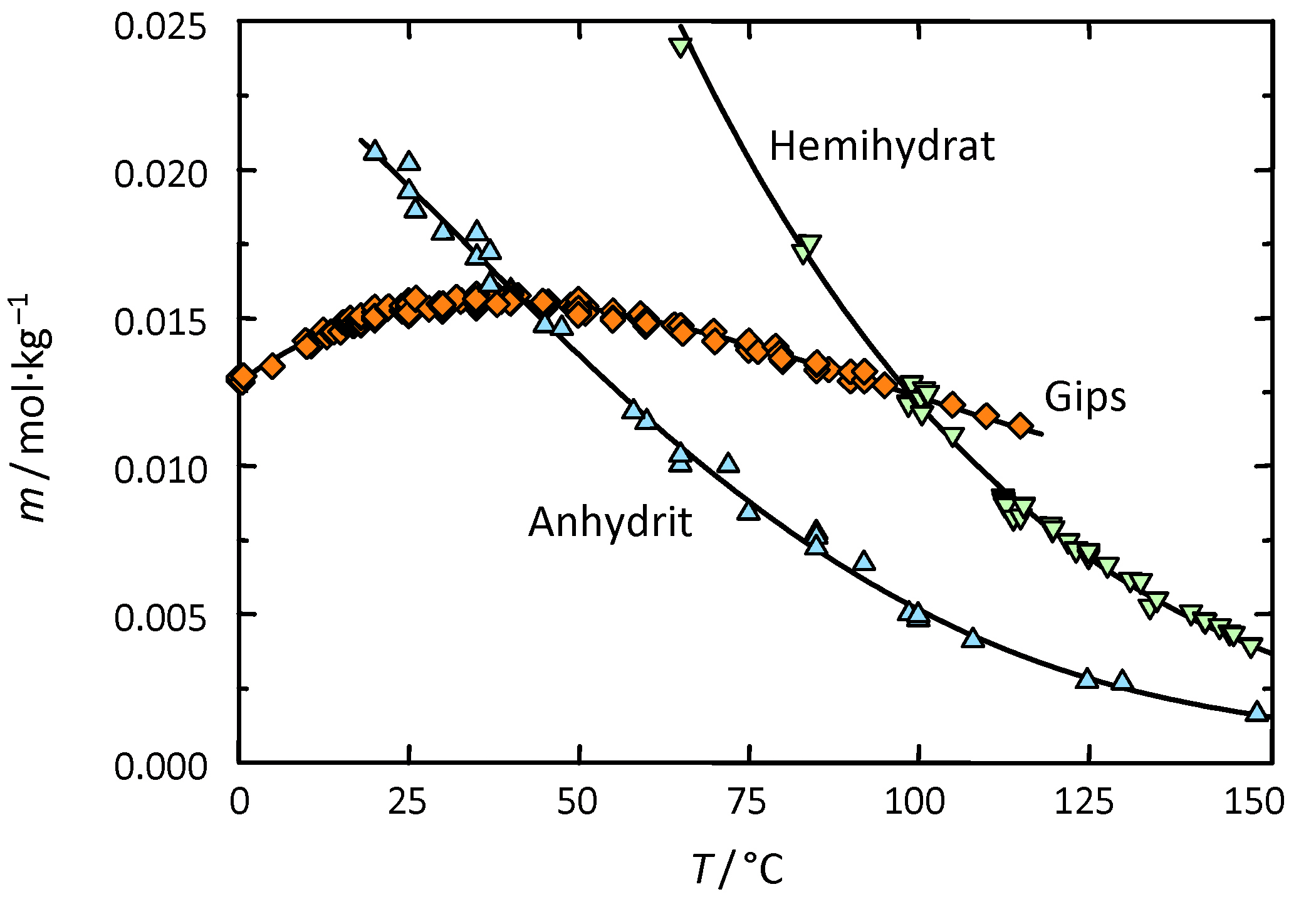
The system CaSO4 – H2O:
calcium sulfate can appear in three different hydrate phases:
- anhydrite- the above mentioned anhydrous form
- hemihydrate- the least stable form
- Gypsum- calcium sulfate dihydrate
Anhydrite exists in different modifications, resulting in different chemical properties, in dependence of the modification (e.g. varying solubility in water). The same applies for hemihydrate.
A value for the transition temperature (in aqueous solution) is the range of between 40°C-66°C. Under normal climatic conditions, on monuments, the precipitation of calcium sulfate from aqueous solution will therefore be predominantly gypsum. Anhydrite forms when the temperature in solution is higher than 40°C-60°C. Parallel to this the formation of large amounts of the metastable hemihydrate takes place during precipitation, it is later transformed into more stable hydrate phases.
When heating the dihydrate (as a solid and dry) to approximately 50°C, the chemically combined water is expelled and hemihydrate forms. However, a complete transition to hemihydrate only takes place at temperatures of around 100°C. If dihydrate is heated to 500-600°C, the anhydrous calcium sulfate is formed. At temperatures above 1000°C the thermal decomposition into calcium oxide and SO3 is effected.
Hygroscopicity[edit]
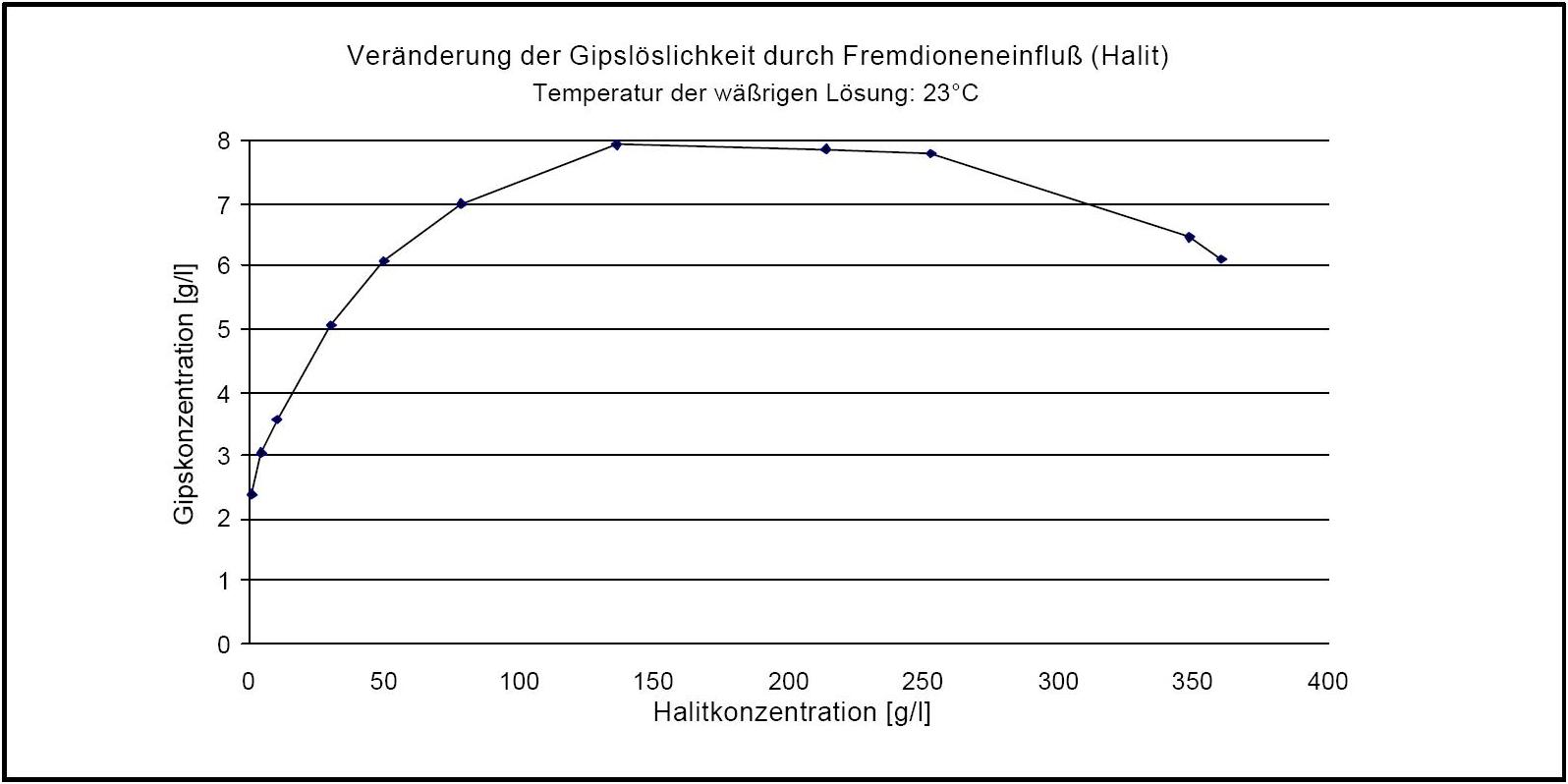
Author: d'Ans, J.
 )
). The pure gypsum salt has no defined Deliquescence point. If, in the presence of halite, relative Humidity levels exceed 90 % RH, gypsum crystals may dissolve, due to the sorption behavior of halite. A decrease of humidity levels to approximately 75% RH causes the recrystallization of gypsum.
Crystallization pressure[edit]
At crystallization in aqueous solution, with the saturation at a ration of 2:1, gypsum produces a linear growth pressure of 28,2-33,4 N/mm2 within a temperature range of 0-50°C. In comparison with other damaging salts, these values lie in the middle range of a calculated scale of values reaching from 7,2 to 65,4 N/mm2 [according to [Winkler:1975]Title: Stone: Properties, Durability in Man´s Environment
Author: Winkler, Erhard M. ].
].
Hydration pressure[edit]
Gypsum as a constituent of an object, can only release the water of crystallization (chemically combined water) at temperatures of approx. 50°C, i.e. it will normally not dehydrate. In contrast the enclosure of the water of crystallization is possible, if anhydrite or hemihydrate are present in a monument. Both processes are associated with a change in volume (of 31.9 % at the conversion from hemihydrate- gypsum) and the emergence of hydration pressure [Zahlenwerte nach [Sperling.etal:1980]Title: Salt Weathering on Arid Environment, I. Theoretical ConsiderationsII. Laboratory Studies
Author: Sperling, C.H.B.and Cooke, R.U. ]. In the instance of a conversion from hemihydrate- gypsum (keyword Gipstreiben) at temperatures ranging from 0-20°C and an RH or 80%, a hydration pressure of 114 –160 N/mm2 can be effected- an extremely high value [ [Stark.etal:1996]Title: Bauschädliche Salze
]. In the instance of a conversion from hemihydrate- gypsum (keyword Gipstreiben) at temperatures ranging from 0-20°C and an RH or 80%, a hydration pressure of 114 –160 N/mm2 can be effected- an extremely high value [ [Stark.etal:1996]Title: Bauschädliche Salze
Author: Stark, Jochen; Stürmer, Sylvia ].
].
Conversion reaction[edit]
The hazardous character of gypsum to historic substance, is connected to the conversion reaction calcite- gypsum. The gypsum molecules formed by calcite hold a volume, that exceeds the volume of the original calcite molecule by about 100%. In this context a relevant damage factor is the modification of the water solubility. Calcite has a water solubility of approx. 0,014g/l (20°C) and is therefore more difficult to dissolve than gypsum. When a conversion to gypsum takes place the result is a more water sensitive system. N.B. The research by Snethlage and Wendler [Snethlage.etal:1998]Title: Steinzerfall und Steinkonservierung - neueste Ergebnisse der Münchner Forschungen
Author: Snethlage, Rolf; Wendler, Eberhard analyses the influence of gypsum on the linear hygroscopic expansion of certain sandstone materials. The damages and the change in swelling behavior of the material was explained through the influence of gypsum.
analyses the influence of gypsum on the linear hygroscopic expansion of certain sandstone materials. The damages and the change in swelling behavior of the material was explained through the influence of gypsum.
Analytical identification[edit]
Microscopy[edit]
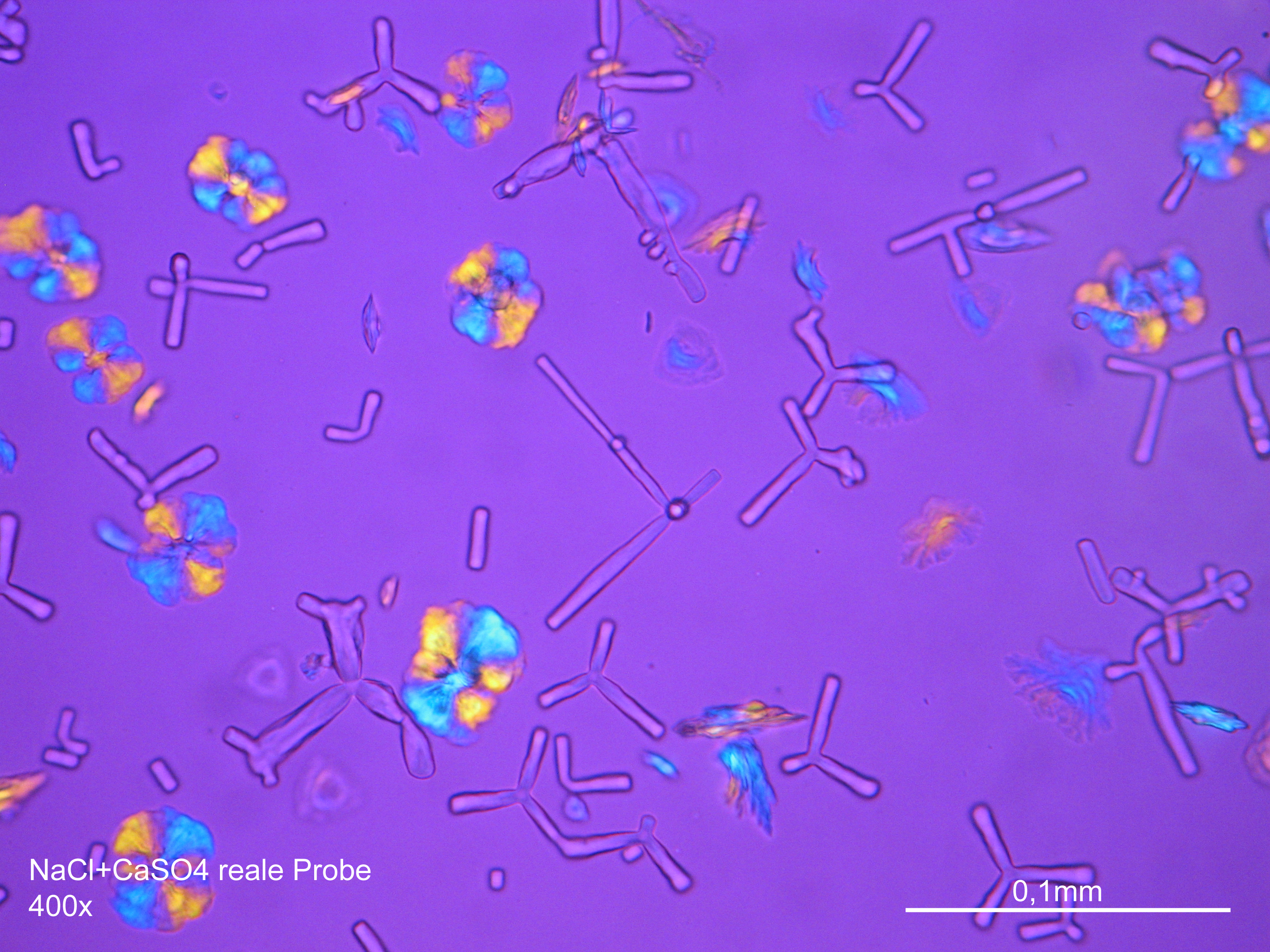
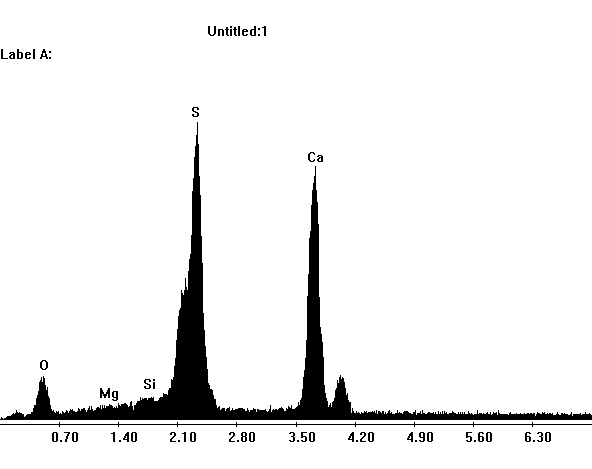
Laboratory examination: Gypsum is slightly water soluble, therefore gypsum-containing sample material only dissolves slightly, when mixed with distilled Water. In solution, gypsum- containing sample material recrystallizes by carefully concentrating the solvent. At first, single needles form, then increasingly needle- like gypsum aggregate in proximity of the seam of the solvent emerges. Alternatively, sample material can be dissolved in hydrochloric acid, which also leads to the formation of crystal needles. Compared to other salts that can recrystallize in needle-like shapes, e.g. sodium carbonate, gypsum needles are clearly shorter.
Refraction indices:
nx = 1.521; ny =1.523; nz =1.530
birefringence: Δ = 0.009
crystal class: monoclinic
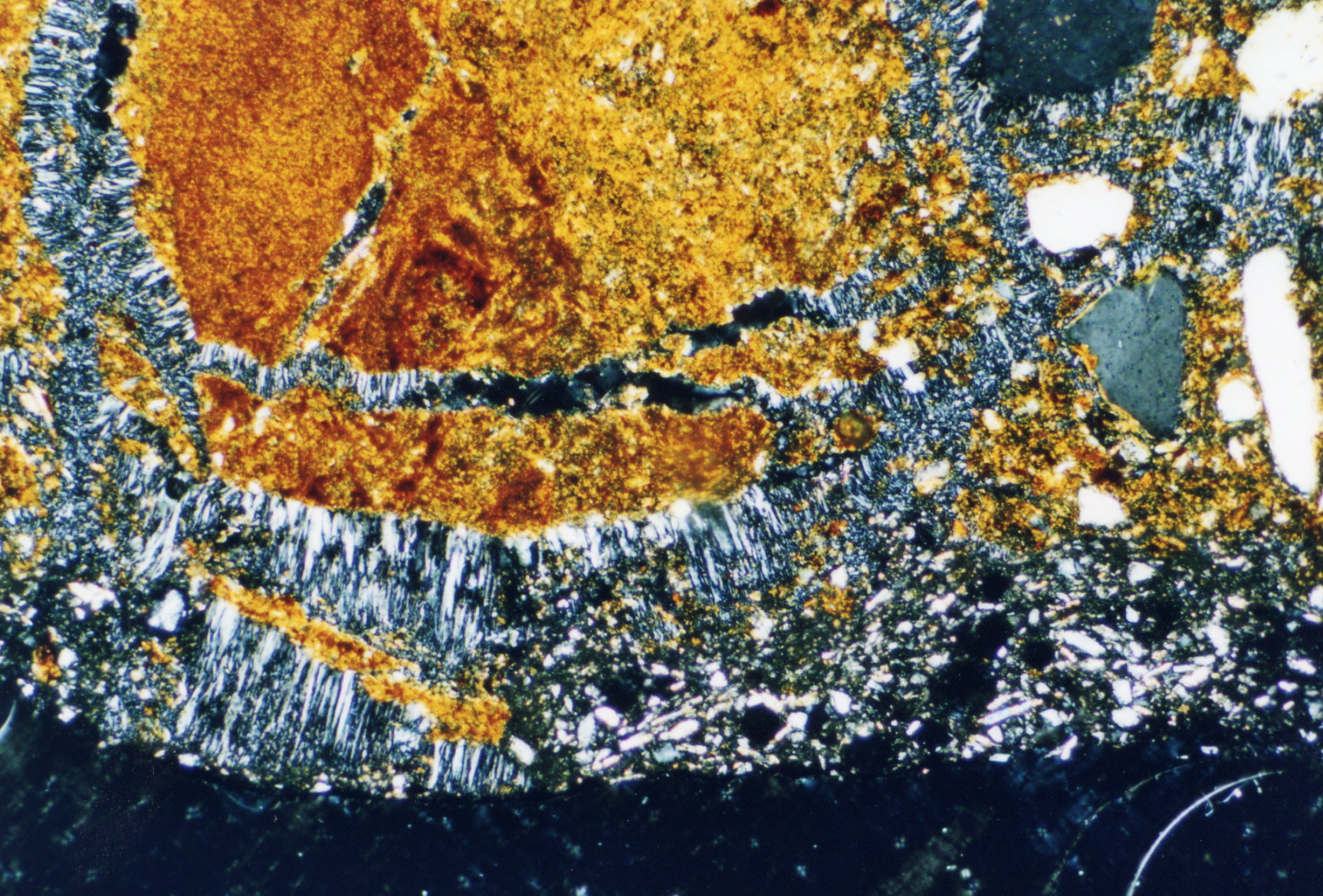
Polarized light microscopy examination:
Apart from the typical acicular habit of gypsum crystals, (especially in recrystallized material) different morphological characteristics appear. These can be useful for identifying gypsum. Gypsum particles (in raw material samples) display shapes of rounded fragments and plate- like rhombohedra, clearly showing the inner cleavage planes. Furthermore, the occurrence of twinning shapes is typical for gypsum crystals, whether they are lath- shaped, tabular or lamellar. The assignment of refractive indices is carried out in accordance with the immersion method using media with indices nD=1,518 und nD=1,53. Due to the often small- scale particles the examination using the Schoeder van der Kolk method is more significant and reliable than the Becke- Line test.
Gypsum crystals belong to the class of monoclinic crystals. Thus, they show, depending on the orientation of the single particle under the microscope, a parallel or respectively a symmetrical extinction, but mainly exhibit a characteristicly oblique axis position in the extinction position. On well developed crystal rhombi the oblique extinction can clearly be measured. Of all calcium sulfate crystals, gypsum has the lowest birefringence. Under crossed polarizers, gypsum has very low interference colors, lying within the gray to yellowish white range of the first order, (of course depending on the thickness of the particles).
Possibility for mistakes:
The Analysis methods mentioned above clearly identify gypsum, provided the following evaluation criteria are explicitly clarified.
- low water solubility
- characteristic needle- like morphology of the recrystallized particles
- all observable indices have a nD –value from 1,518 and 1,530
- gypsum crystal show low interference colors
- gypsum crystals have an oblique extinction
| salt phase | differentiating features |
| Syngenite K2Ca(SO4) • 2H2O | all observable indices; 1,518 |
| Tachyhydrite CaMg2Cl6 • 12H2O | mostly observable index < 1,518 / only parallel and symmetrical extinction |
| Hydromagnesite Mg5[OH(CO3)2]2 • 4H2O | mostly one index > 1,53 |
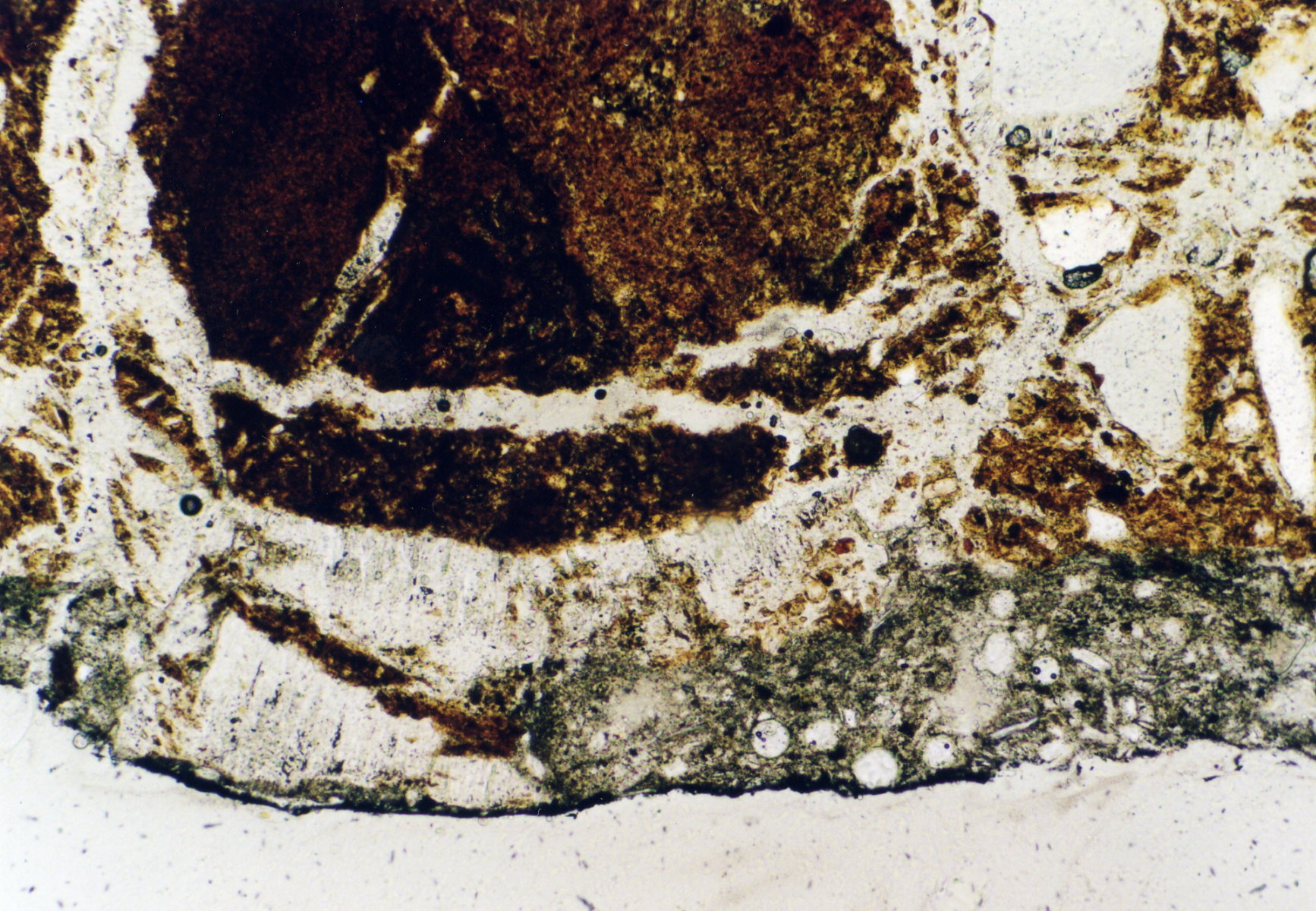
Photos of gypsum crystals and deterioration pattern caused by gypsum[edit]
On an object[edit]
Under the polarising microscope[edit]
- In thin section of bricks
- Gypsum crystallized out of a solution in water on a glass slide
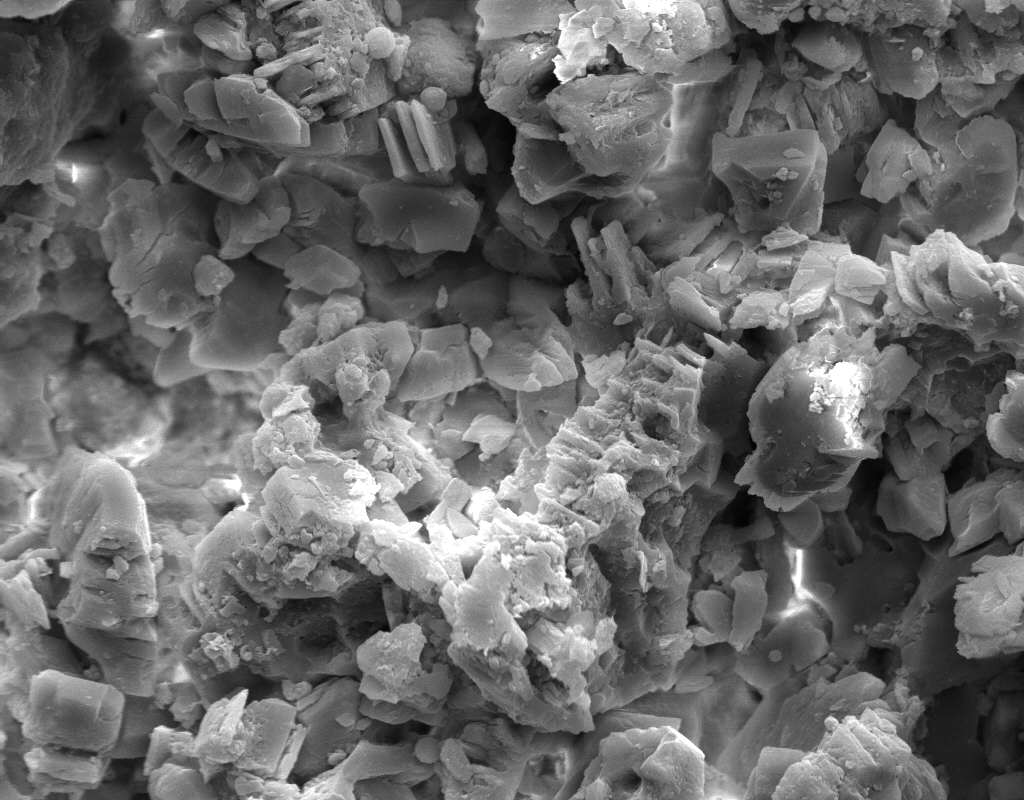
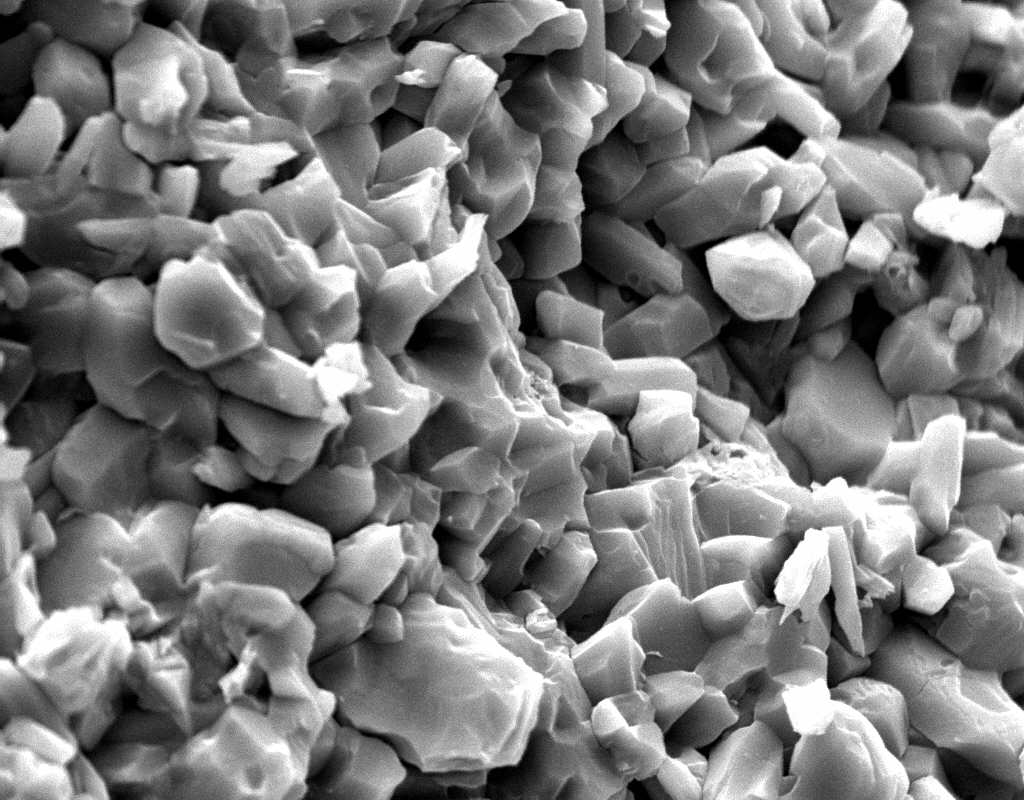
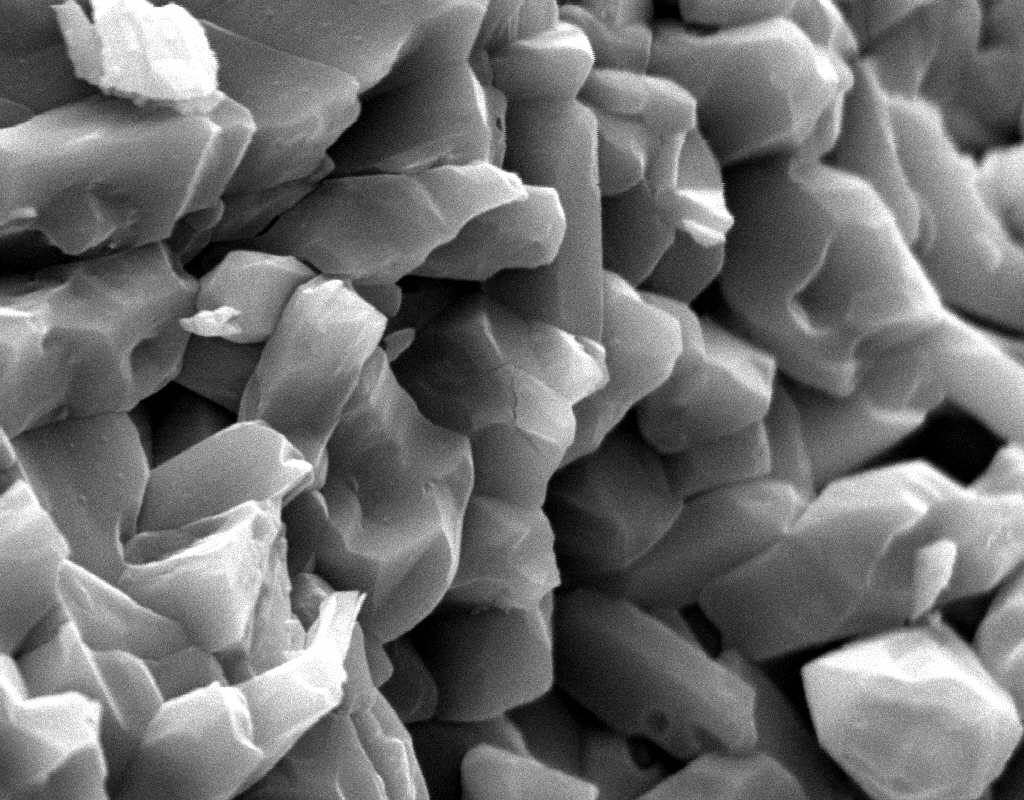
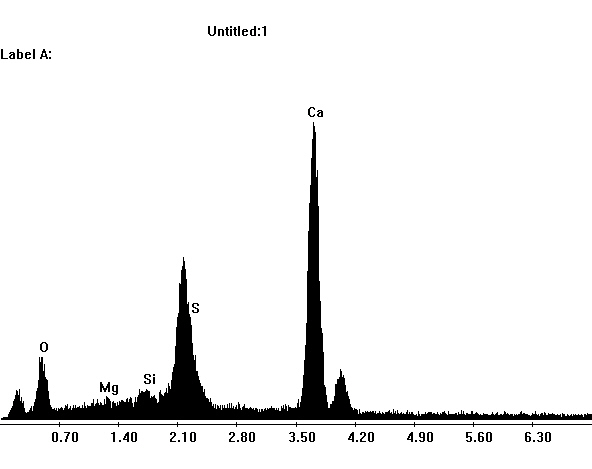
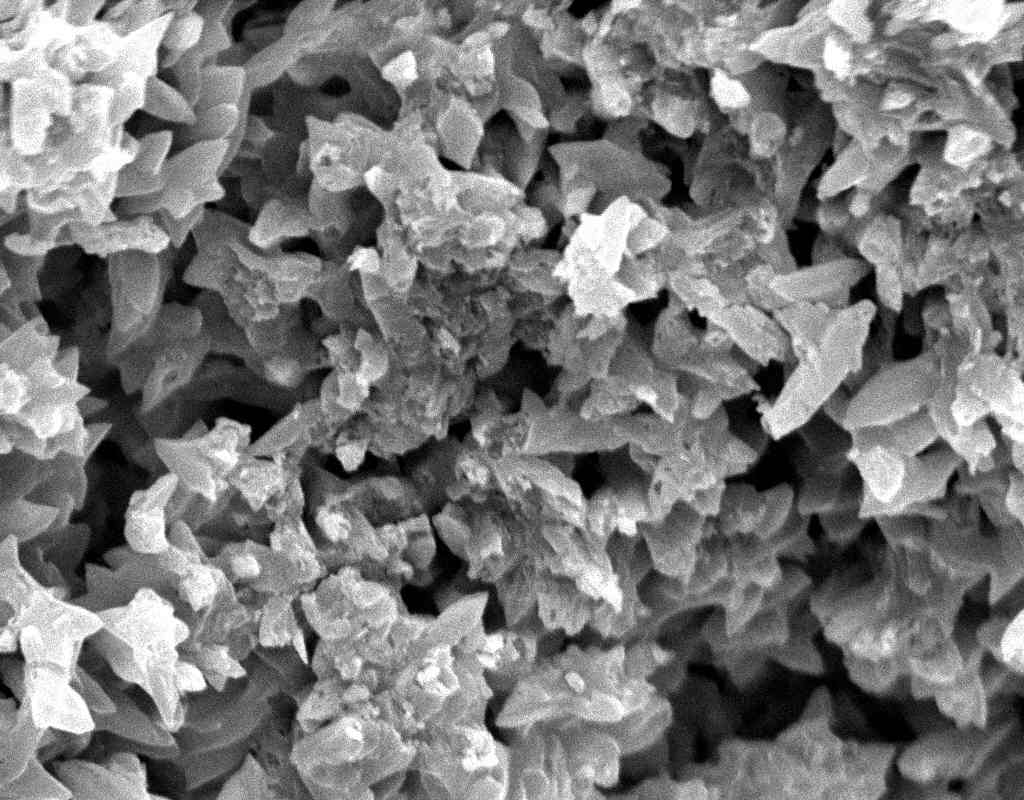
Under the Scanning Electron Microscope (SEM)[edit]
- In a SEM
Literatur[edit]
| [DAns:1933] | d'Ans, J. (1933): Die Lösungsgleichgewichte der Systeme der Salze ozeanischer Salzablagerungen, Verlagsgesellschaft für Ackerbau, M.B.H. Berlin |  |
| [Dana:1951] | Dana E.S. (eds.) Dana J.D. (1951): Dana's System of Mineralogy, 7, Wiley & Sons |  |
| [Robie.etal:1978] | Robie R.A., Hemingway B.S.; Fisher J.A. (1978): Thermodynamic properties of minerals and related substances at 298.15 K and 1 bar pressure and higher temperatures. In: U.S. Geol. Surv. Bull, 1452 () |  |
| [Snethlage.etal:1998] | Snethlage, Rolf; Wendler, Eberhard (1998): Steinzerfall und Steinkonservierung - neueste Ergebnisse der Münchner Forschungen. In: Münchner Geologische Hefte, A 23, Festschrift zum 65. Geburtstag von Prof. Dr. Dietrich D. Klemm, (), 177-201 |  |
| [Sperling.etal:1980] | Sperling, C.H.B.and Cooke, R.U. (1980): Salt Weathering on Arid Environment, I. Theoretical ConsiderationsII. Laboratory Studies. In: Papers in Geography, 8 () |  |
| [Stark.etal:1996] | Stark, Jochen; Stürmer, Sylvia (1996): Bauschädliche Salze, Bauhaus-Univ. Weimar |  |
| [Winkler:1975] | Winkler, Erhard M. (1975): Stone: Properties, Durability in Man´s Environment, Springer Verlag, Wien |  |
Weblinks[edit]
- ↑ http://webmineral.com/data/Gypsum.shtml seen on 30.07.2010
- ↑ http://www.mindat.org/min-1784.html seen on 30.07.2010