Calcium chloride: Difference between revisions
Jump to navigation
Jump to search
No edit summary |
|||
| Line 17: | Line 17: | ||
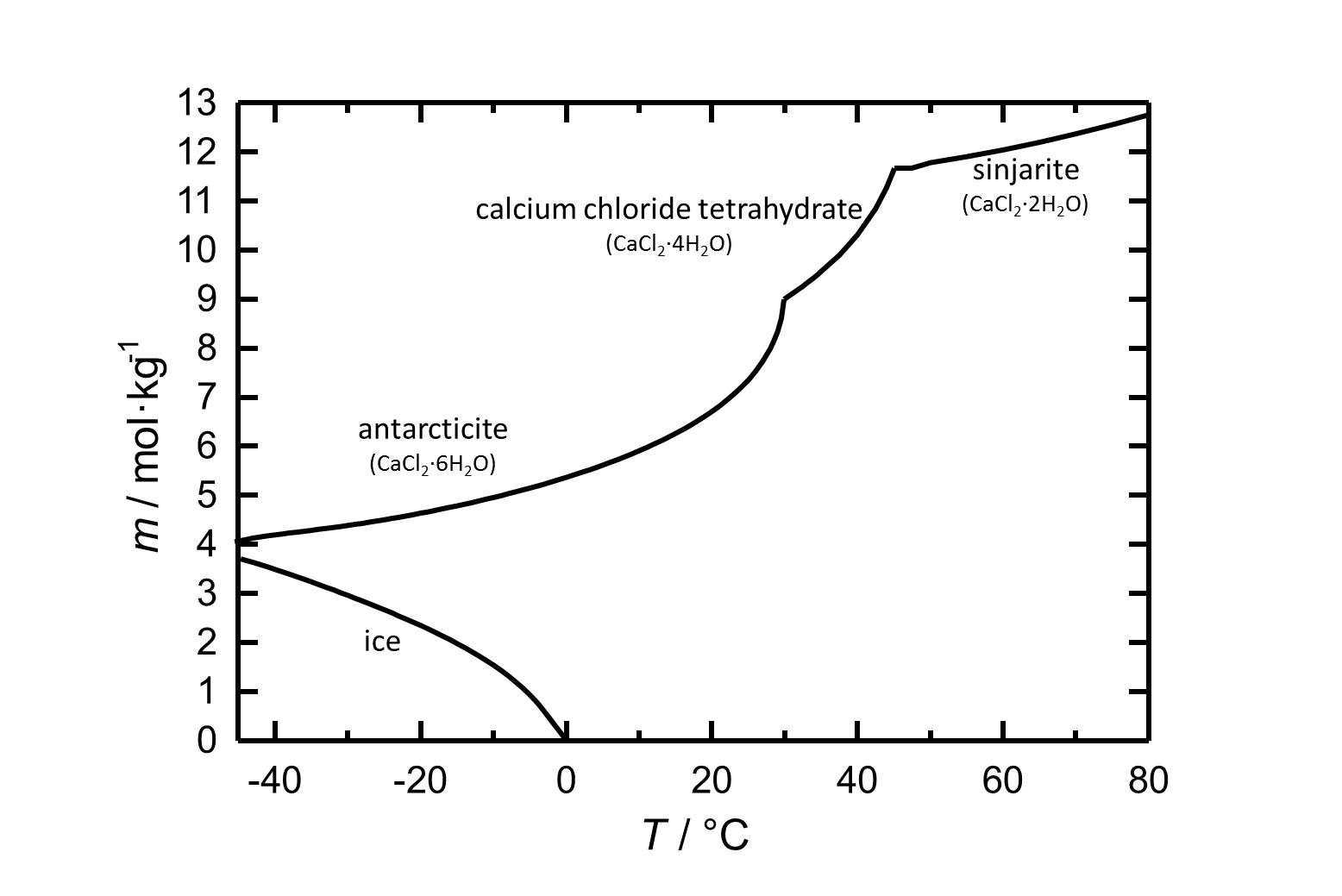
[[file:L CaCl2 d.jpg|thumb|left|800px|Figure 1: Solubility of calcium chloride in water. The molality ''m'' [n(CaCl<sub>2</sub>•xH<sub>2</sub>O)•kg(H<sub>2</sub>O)<sup>-1</sup>] is plotted against the temperature.]] | [[file:L CaCl2 d.jpg|thumb|left|800px|Figure 1: Solubility of calcium chloride in water. The molality ''m'' [n(CaCl<sub>2</sub>•xH<sub>2</sub>O)•kg(H<sub>2</sub>O)<sup>-1</sup>] is plotted against the temperature.]] | ||
<br clear=all> | <br clear=all> | ||
Under standard conditions the hexahydrate of calcium chloride [[Antarcticite]] is the stable form. The salt has got a high solubility which increases with increasing temperatures. The dehydration steps to the [[calcium chloride tetrahydrate]] and to [[sinjarite]] take place at temperatures of 30 °C and 45 °C, respectively. | |||
==Hygroscopicity== | ==Hygroscopicity== | ||
Revision as of 14:00, 25 February 2015
Author: Amelie Stahlbuhk
back to Chloride
| This article will be released soon. |
Abstract[edit]
The different hydrate stages of calcium chloride are presented, as well as their behavior regarding solubility and hygroscopicity.
Hydrate stages[edit]
Sinjarite: CaCl2•2H2O
Calcium chloride tetrahydrate: CaCl2•4H2O
Antarcticite: CaCl2•6H2O
Solubility[edit]
Under standard conditions the hexahydrate of calcium chloride Antarcticite is the stable form. The salt has got a high solubility which increases with increasing temperatures. The dehydration steps to the calcium chloride tetrahydrate and to sinjarite take place at temperatures of 30 °C and 45 °C, respectively.
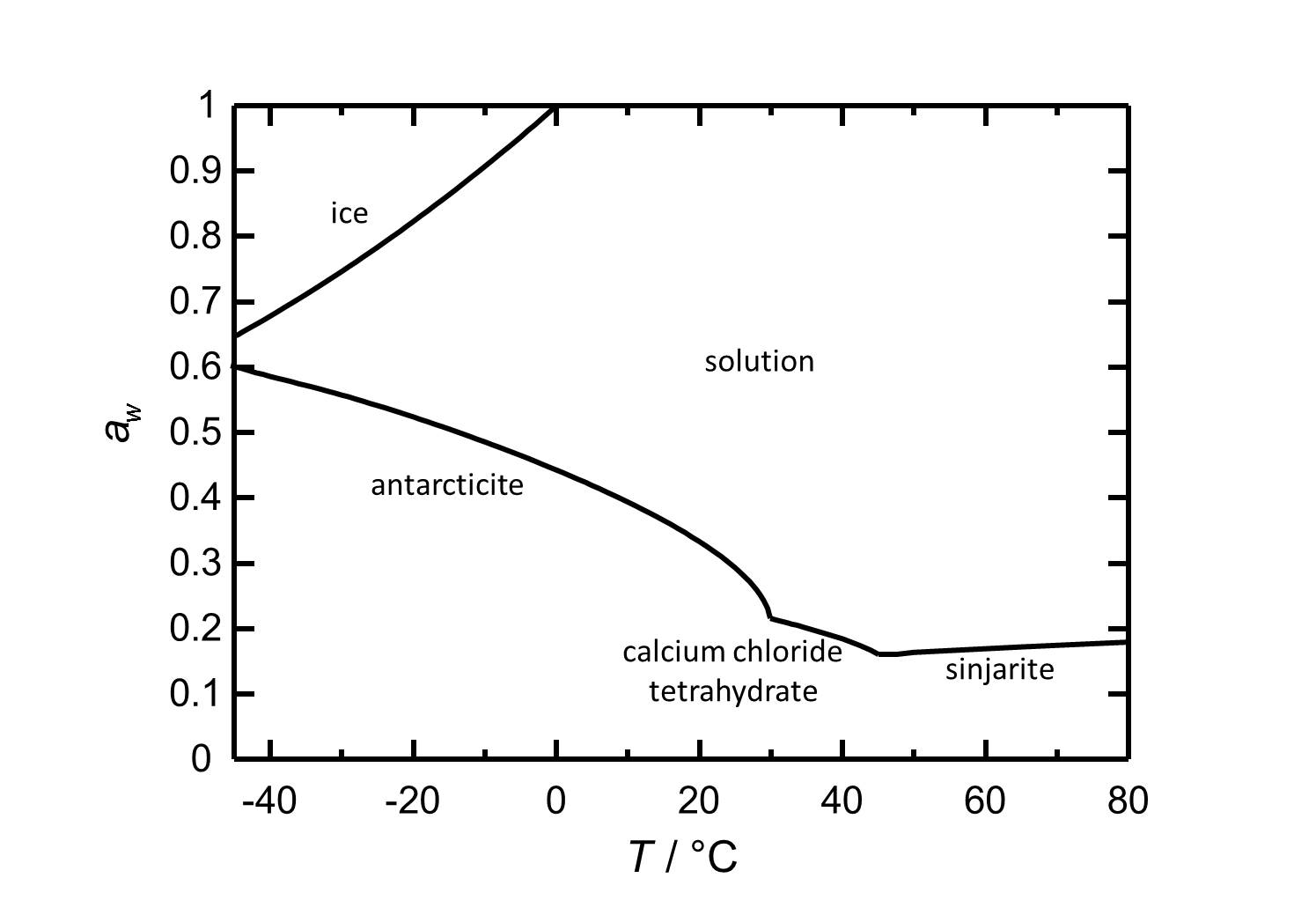
Hygroscopicity[edit]