Natrite: Difference between revisions
Jump to navigation
Jump to search
No edit summary |
No edit summary |
||
| Line 9: | Line 9: | ||
|Crystal_System =monoclinic | |Crystal_System =monoclinic | ||
|Crystal_Structure = | |Crystal_Structure = | ||
|Deliqueszenzhumidity = | |Deliqueszenzhumidity = | ||
|Solubility = | |Solubility =210 g/l | ||
|Density =2,532 g/cm<sup>3</sup> | |Density =2,532 g/cm<sup>3</sup> | ||
|MolVolume = | |MolVolume = | ||
| Line 25: | Line 25: | ||
|Phase_Transition = | |Phase_Transition = | ||
|chemBehavior = | |chemBehavior = | ||
|Comments = | |Comments =blooms in dry air and converts to the monohydrate<br>pH about 12 | ||
}} | }} | ||
Revision as of 12:43, 9 July 2011
| Natrite[1][2] | |
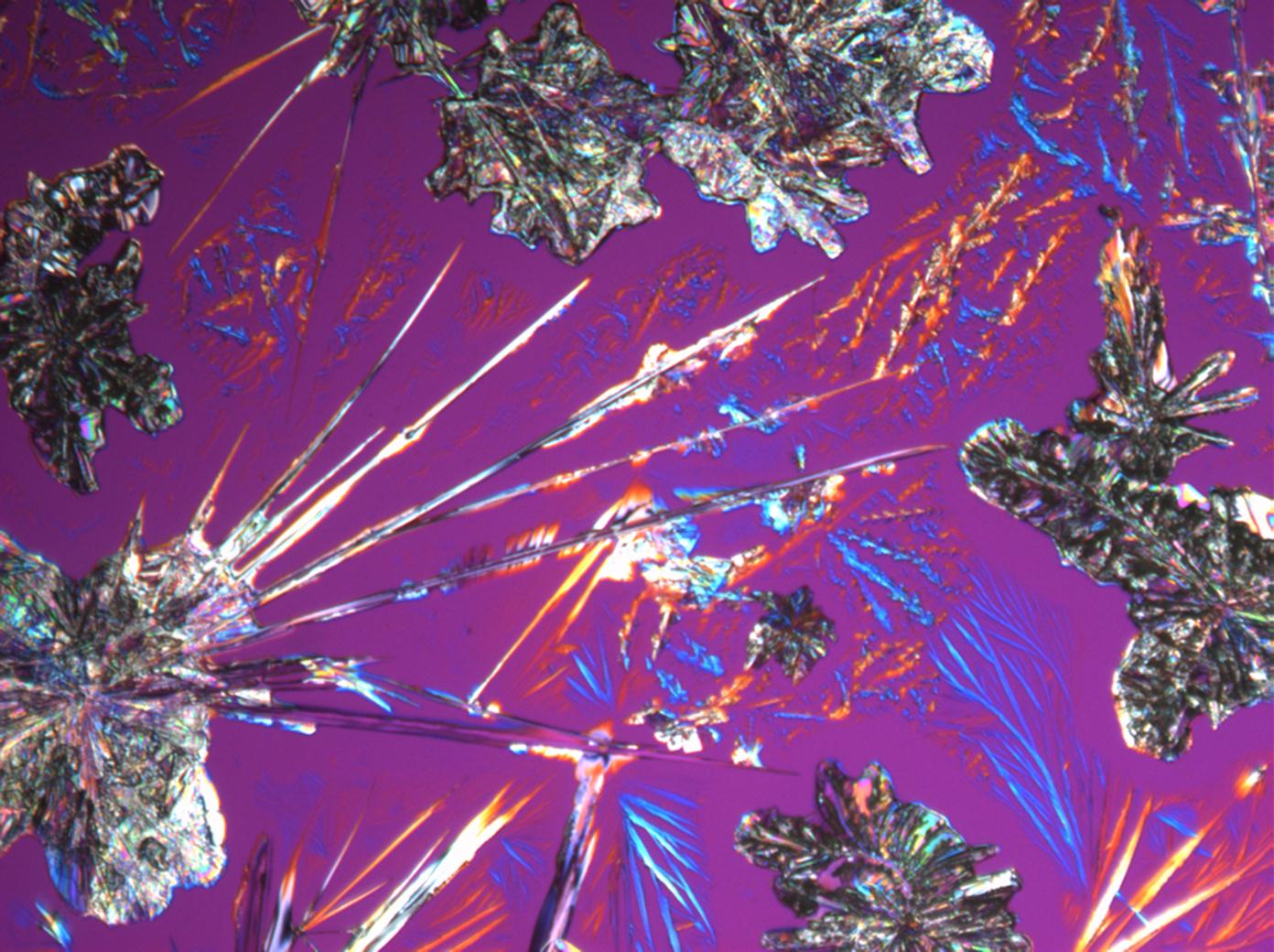
| |
| Mineralogical name | Natrite |
| Chemical name | Sodium carbonate |
| Trivial name | |
| Chemical formula | Na2CO3 |
| Other forms | Sodium carbonate hydrate (Na2CO3•H2O) Sodium carbonate heptahydrate (Na2CO3•7H2O) Sodium carbonate decahydrate (Na2CO3•10H2O) |
| Crystal system | monoclinic |
| Crystal structure | |
| Deliquescence humidity 20°C | |
| Solubility (g/l) at 20°C | 210 g/l |
| Density (g/cm³) | 2,532 g/cm3 |
| Molar volume | |
| Molar weight | 105,99 g/mol |
| Transparency | |
| Cleavage | |
| Crystal habit | |
| Twinning | |
| Phase transition | |
| Chemical behavior | |
| Comments | blooms in dry air and converts to the monohydrate pH about 12 |
| Crystal Optics | |
| Refractive Indices | nx = 1,415 ny = 1,535 nz = 1,546 |
| Birefringence | Δ = 0,131 |
| Optical Orientation | biaxial negative |
| Pleochroism | |
| Dispersion | |
| Used Literature | |
| {{{Literature}}} | |
back to Carbonate
Weblinks[edit]
- ↑ http://webmineral.com/data/Natrite.shtml viewed on 09/06/2011
- ↑ http://www.mindat.org/min-2849.html viewed on 09/06/2011