Halite: Difference between revisions
No edit summary |
CGerdwilker (talk | contribs) No edit summary |
||
| Line 1: | Line 1: | ||
<bibimport /> | |||
Autoren: [[Benutzer:Hschwarz|Hans-Jürgen Schwarz]], Nils Mainusch | |||
<br>English version by Christa Gerdwilker | |||
<br>zurück zu [[Chloride]] | |||
{{Infobox_Salt | {{Infobox_Salt | ||
|Footnote =<ref>http://webmineral.com/data/Halite.shtml viewed on 28/07/2010</ref><ref>http://www.mindat.org/min-1804.html viewed on 28/07/2010</ref> | |Footnote =<ref>http://webmineral.com/data/Halite.shtml viewed on 28/07/2010</ref><ref>http://www.mindat.org/min-1804.html viewed on 28/07/2010</ref> | ||
| Line 16: | Line 22: | ||
|Transparency =transparent to translucent | |Transparency =transparent to translucent | ||
|Cleavage =perfect | |Cleavage =perfect | ||
|Crystal_Habit = | |Crystal_Habit =cubic crystal, granular, massive aggregates | ||
|Twinning = | |Twinning =none | ||
|Refractive_Indices =n=1.544 | |Refractive_Indices =n=1.544 | ||
|Birefringence = | |Birefringence = | ||
| Line 25: | Line 31: | ||
|Phase_Transition = | |Phase_Transition = | ||
|chemBehavior = | |chemBehavior = | ||
|Comments = | |Comments =slightly watersoluble | ||
}} | }} | ||
== Abstract == | |||
== Halite origins == | |||
Sodium chloride is obtained through mining or derived from the sea or salt lakes and is commonly used for cooking or as road gritting salt. <br> | |||
The salt content of sea water is approx. 2,7 M.%.<br> | |||
== Causes of halite formation on monuments == | |||
Contact with materials containing soluble sodium-based ingredients can result in the efflorescence of sodium chloride on monuments. A primary example is the high sodium content of cements. Contamination with sodium and chloride ions can also occur through contact with salt laden ground or surface water. A range of cleaning materials (e.g. acidic and caustic cleaners) and especially previously used restoration materials (e.g. water glass) can introduce sodium and chloride ions into monuments. Further common sources of halite are rock salt used in road gritting which predominantly consists of sodium chloride, as well as salt laden seawater in coastal areas.<br> | |||
<br clear=all> | |||
== Solubility behavior == | |||
The commonly occurring halite found in northern Germany has a solubility of 358 g/l (20°C) and thus belongs to the group of very soluble and, therefore, easily mobilized salts. Its solubility changes comparatively little within a temperature range of 10 -30°C. | |||
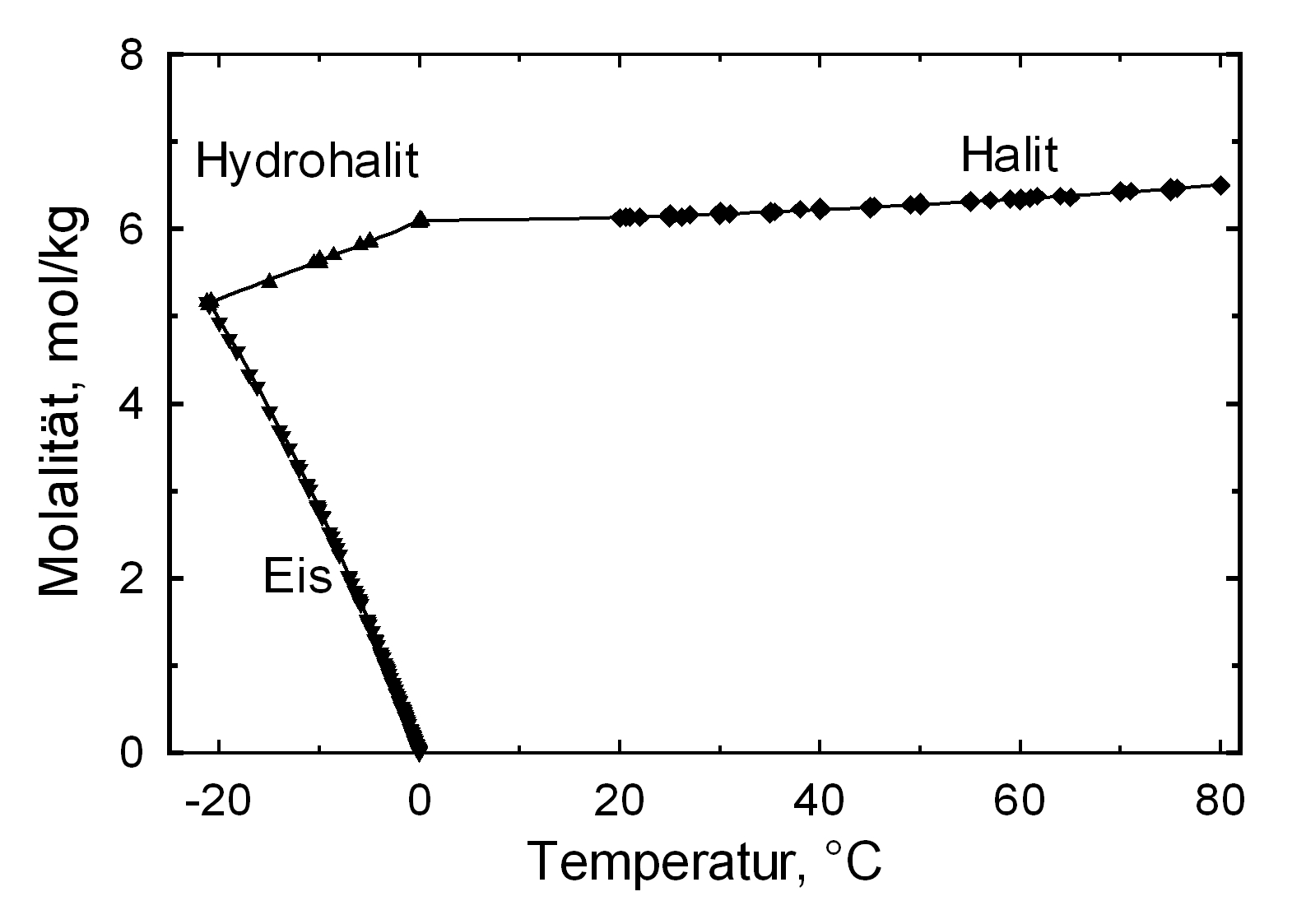
[[Image:NaCl s.jpg|thumb|right|400px|Image 1:Phase diagram of halite. Graphic: Michael Steiger]] | |||
<br clear="all"> | |||
{|border="2" cellspacing="0" cellpadding="4" width="52%" align="left" class="wikitable" | |||
|+''Table 1: Solubility of halite in relation to temperature [after <bib id="Stark.etal:1996" /> und <bib id="DAns:1933" /> '' | |||
|- | |||
|bgcolor = "#F0F0F0"| '''Temperature''' | |||
|bgcolor = "#F0F0F0" align=center| '''10°C''' | |||
|bgcolor = "#F0F0F0" align=center| '''20°C''' | |||
|bgcolor = "#F0F0F0" align=center| '''40°C''' | |||
|- | |||
|bgcolor = "#F7F7F7" | Solubility [g/l] | |||
|bgcolor = "#FFFFEO" align=center| 356,5 | |||
|bgcolor = "#FFFFEO" align=center| 358,8 | |||
|bgcolor = "#FFFFEO" align=center| 364,2 | |||
|} | |||
<br clear=all> | |||
== Hygroscopicity == | |||
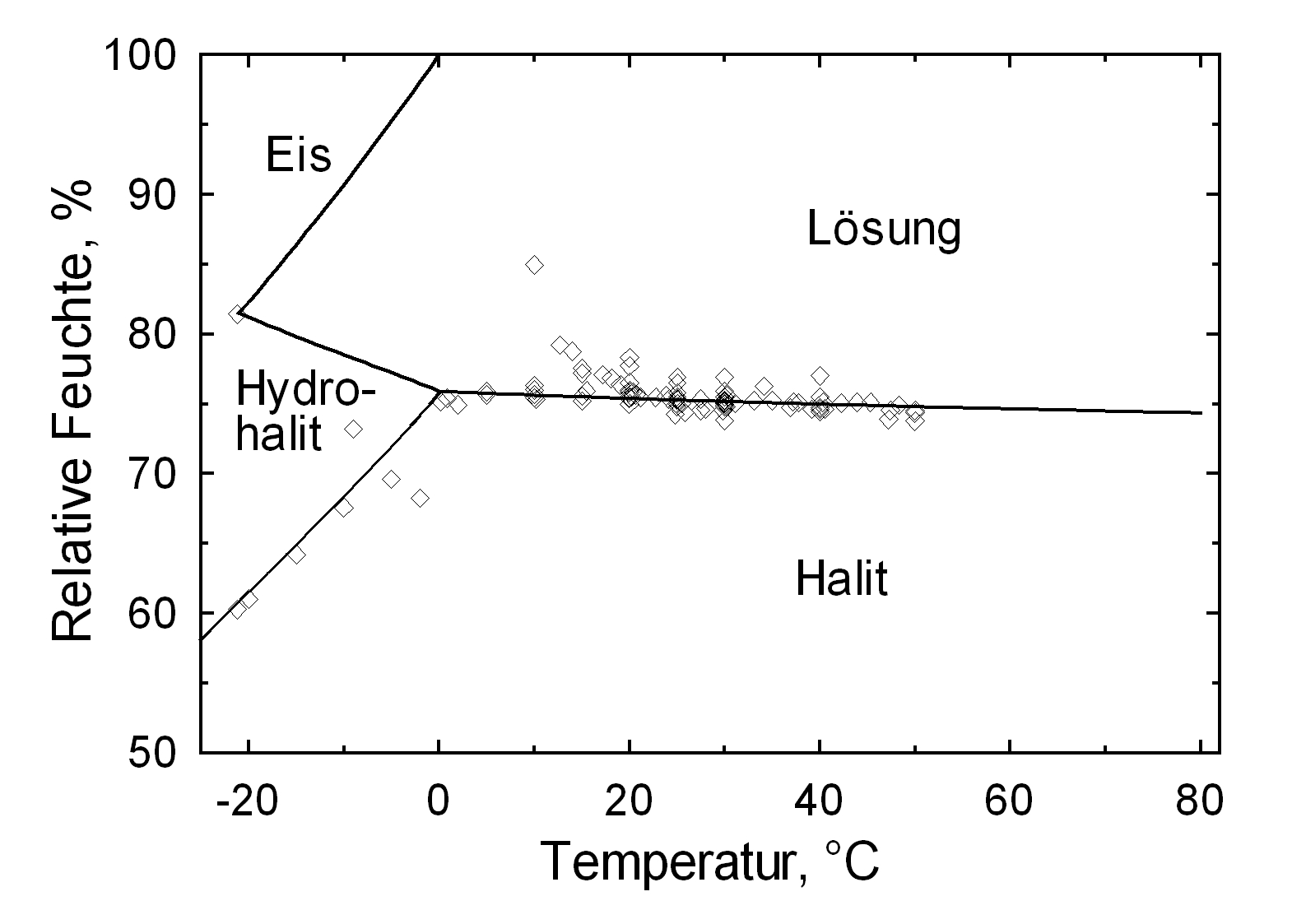
[[Image:NaCl a.jpg|thumb|right|400px|Image 3: The system NaCl/H<sub>2</sub>O within temperature range of -20°C to 80°C. Graphic: Michael Steiger]] | |||
Halite’s deliquescence humidity of approx. 75% is often crossed in the climate of northern Europe. Temperature variations hardly affect the deliquescence point of halite which is illustrated below in comparison with potassium nitrate and natrite. | |||
<br> '''Moisture sorption:'''<br>Theoretically 1g NaCl can absorb 4,3g moisture. The moisture sorption during varying relative humidity levels is: | |||
<br clear="all"> | |||
{|border="2" cellspacing="0" cellpadding="4" width="52%" align="left" class="wikitable sortable" | |||
|+''Tabelle 2:Moisture sorption in M% after 56 days according to <bib id=Vogt.etal:1993/> '' | |||
|- | |||
|bgcolor = "#F0F0F0"| '''Relative humidity during storge/salt phase''' | |||
|bgcolor = "#F0F0F0" align=center| '''NaCl''' | |||
|- | |||
|bgcolor = "#F7F7F7" | '''87% RH''' | |||
|bgcolor = "#FFFFEO" align=center| 153 | |||
|- | |||
|bgcolor = "#F7F7F7" | '''81% RH''' | |||
|bgcolor = "#FFFFEO" align=center| 22 | |||
|- | |||
|bgcolor = "#F7F7F7" | '''79% RH''' | |||
|bgcolor = "#FFFFEO" align=center| 7 | |||
|} | |||
<br clear=all> | |||
== Crystallization pressure == | |||
The crystallization of halite from a water-based solution results in a crystallization pressure of 55,4-65,4 N/mm<sup>2</sup> <bib id="Winkler:1975" /> (for comparison, the crystallization pressure of different salts can range from 7,2-65,4 N/mm<sup>2</sup>). These values need to be considered in conjunction with temperature and concentration and can, therefore, only act as indicators of damage potential in relation to salt crystallization pressure. In comparison to other salts, the crystallization pressure of halite is extremely high. | |||
== Hydration behavior == | |||
Under normal conditions only the unhydrated form of halite exists. Only at a temperature of below 0.15 °C does a saturated water-based sodium chloride solution result in the precipitation of a deposit of dihydrate hydrohalite<ref name=hydrohalit>http://www.mineralienatlas.de/lexikon/index.php/MineralData?mineral=Hydrohalit accessed 28.07.2010</ref>. | |||
<!-- | |||
=== Hydration pressure === | |||
=== Transformation reactions === | |||
== <br>Analytical validation == | |||
--> | |||
== Microscopy == | |||
=== Laboratory examinations === | |||
Sodium chloride crystals can be reliably identified on the basis of morphological features. Individual particles usually form cubic or octahedral shapes and, therefore, clearly display right angles in their crystal construction. <br> | |||
'''Refractive index:''' n<sub>D</sub> = 1,544<br>'''Crystal category:''' cubic<br> | |||
'''Examinations under the polarising microscope:''' | |||
Together with potassium chloride, sodium chloride is one of the few salts belonging to the cubic crystal system which cause damage to masonry. Because of its isotropic internal structure it does not display birefringence. | |||
The classification of the refractive index occurs by immersion method in a standard oil with a refractive index of n<sub>D</sub> =1,518. Halite crystals display the same optical density in every direction so that the speed and orientation of linear polarised light waves are not distorted. When viewed between crossed polars, the crystals are not visible but appear (independent of orientation) extinguished. | |||
<br> '''Differentiating of halite from similar salts:'''<br> | |||
The group of isotropic salts causing masonry damage consists of halite, sylvine and fluorite. All these phases can be differenciated easily. | |||
<br clear="all"> | |||
{|border="2" cellspacing="0" cellpadding="4" width="50%" align="left" class="wikitable" | |||
|+''Table 3: Identification features of other chlorides'' | |||
|- | |||
{| cellspacing="1" cellpadding="1" border="1" style="width: 498px; height: 85px;" | |||
|- | |||
|bgcolor = "#F0F0F0"| '''Salt phase''' | |||
|bgcolor = "#F0F0F0"| '''Identification features''' | |||
|- | |||
|bgcolor = "#F7F7F7"| [[Sylvine]] KCl | |||
|bgcolor = "#FFFFEO"| Refractive index under 1,518. | |||
|- | |||
|bgcolor = "#F7F7F7"| [[Fluorite]] CaF<sub>2</sub> | |||
|bgcolor = "#FFFFEO"| Refractive index under 1,518, barely water soluble. | |||
|} | |||
<!-- | |||
=== <br>X-ray diffraction === | |||
=== <br>Raman-Spectroscopy == | |||
=== DTA / TG === | |||
=== <br>IR- Spectroscopy === | |||
== <br>Dealing with halite damage == | |||
--> | |||
== Images of salts and salt damage == | |||
=== On the object === | |||
<gallery caption="" widths="200px" heights="150px" perrow="3"> | |||
Image:Strahlsund_Kochsalz-Kruste.jpg|Halite crust on a brick surface Image:Strahlsund_Kochsalz-Whisker.jpg|Halite Whisker | |||
</gallery> | |||
=== Under the polarising microscope === | |||
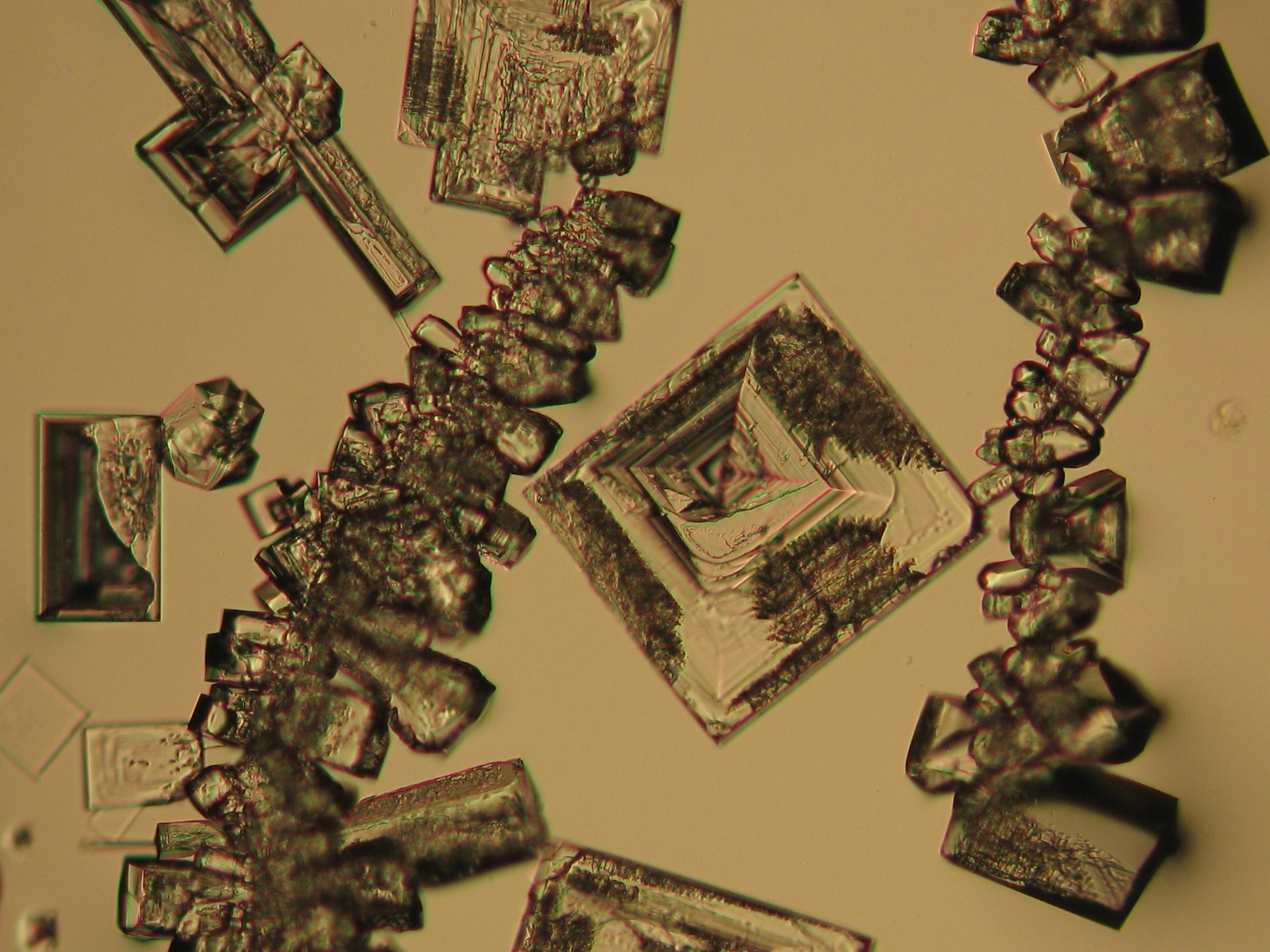
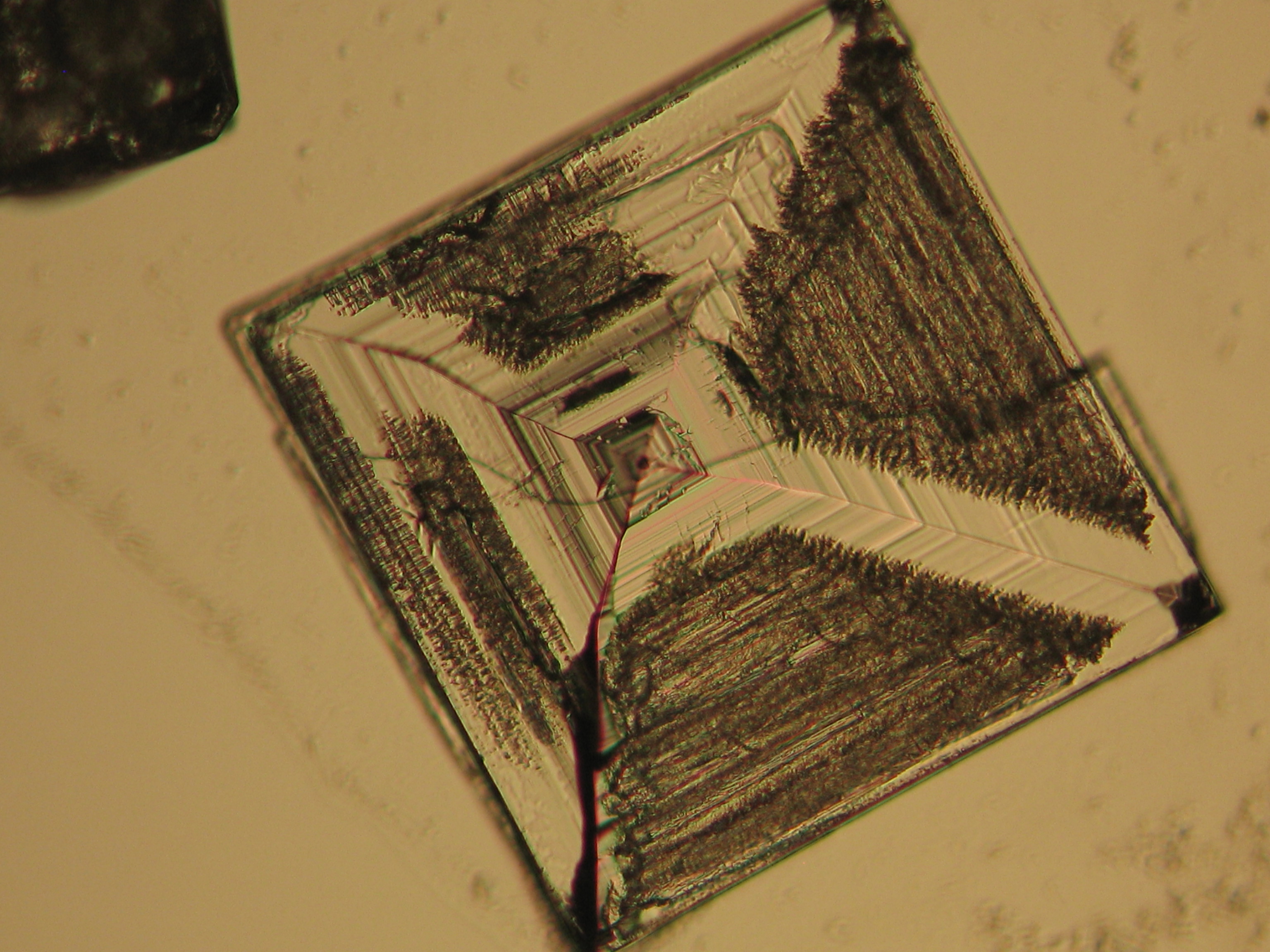
<gallery caption="Precipitate from watery sample on microscope slide" widths="200px" heights="150px" perrow="3"> | |||
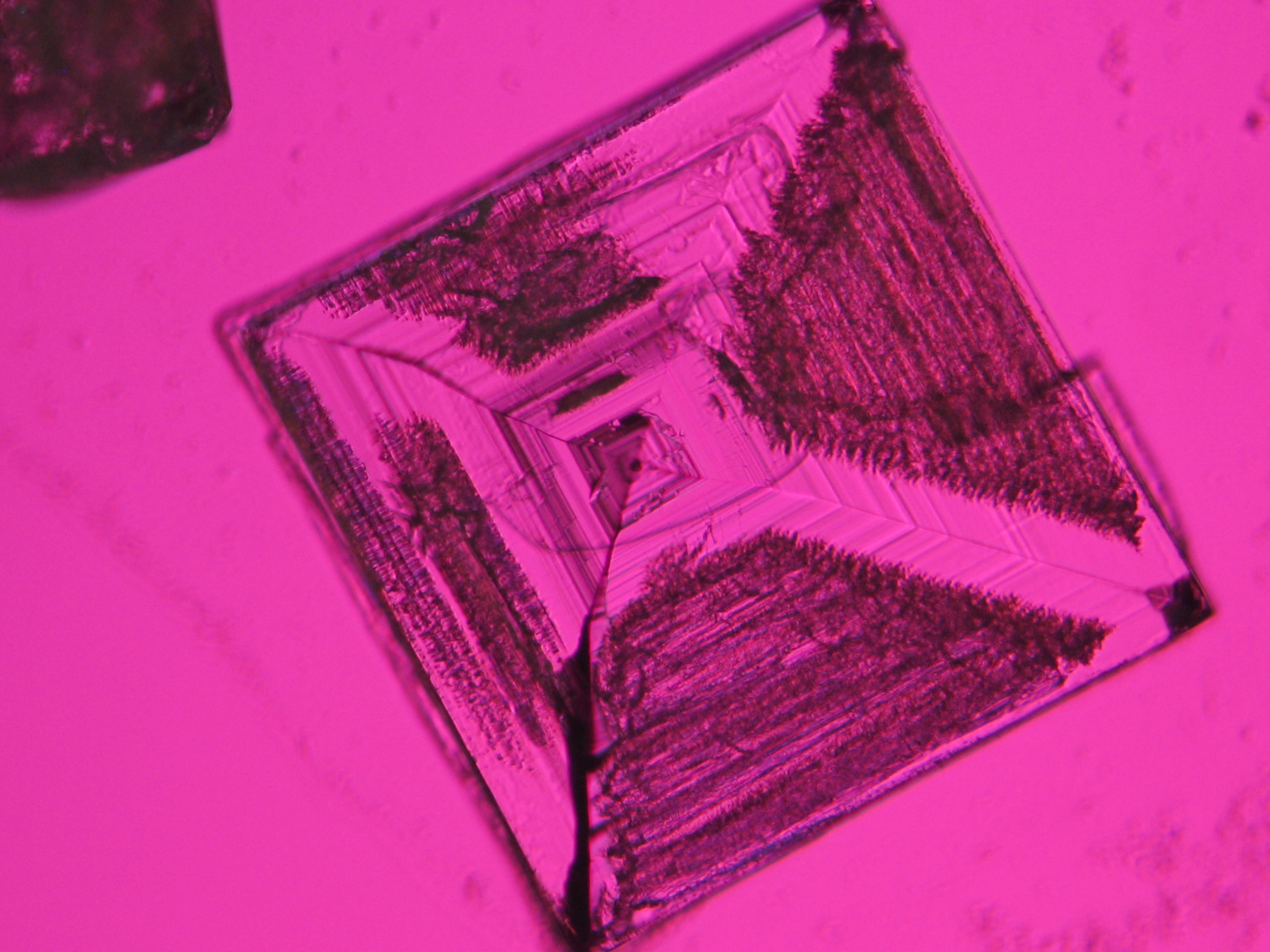
Image:NaCl 27.4.2006-10x (1).JPG|Sodium chloride, precipitate from watery sample on microscope slide | |||
Image:NaCl 27.4.2006-10x (2).JPG|Sodium chloride, precipitate from watery sample on microscope slide | |||
Image:NaCl 27.4.2006-10x (5).JPG|Sodium chloride, precipitate from watery sample on microscope slide | |||
Image:NaCl 27.4.2006-10x (6).JPG|Sodium chloride, precipitate from watery sample on microscope slide | |||
Image:NaCl 27.4.2006-10x.JPG| Sodium chloride, precipitate from watery sample on microscope slide | |||
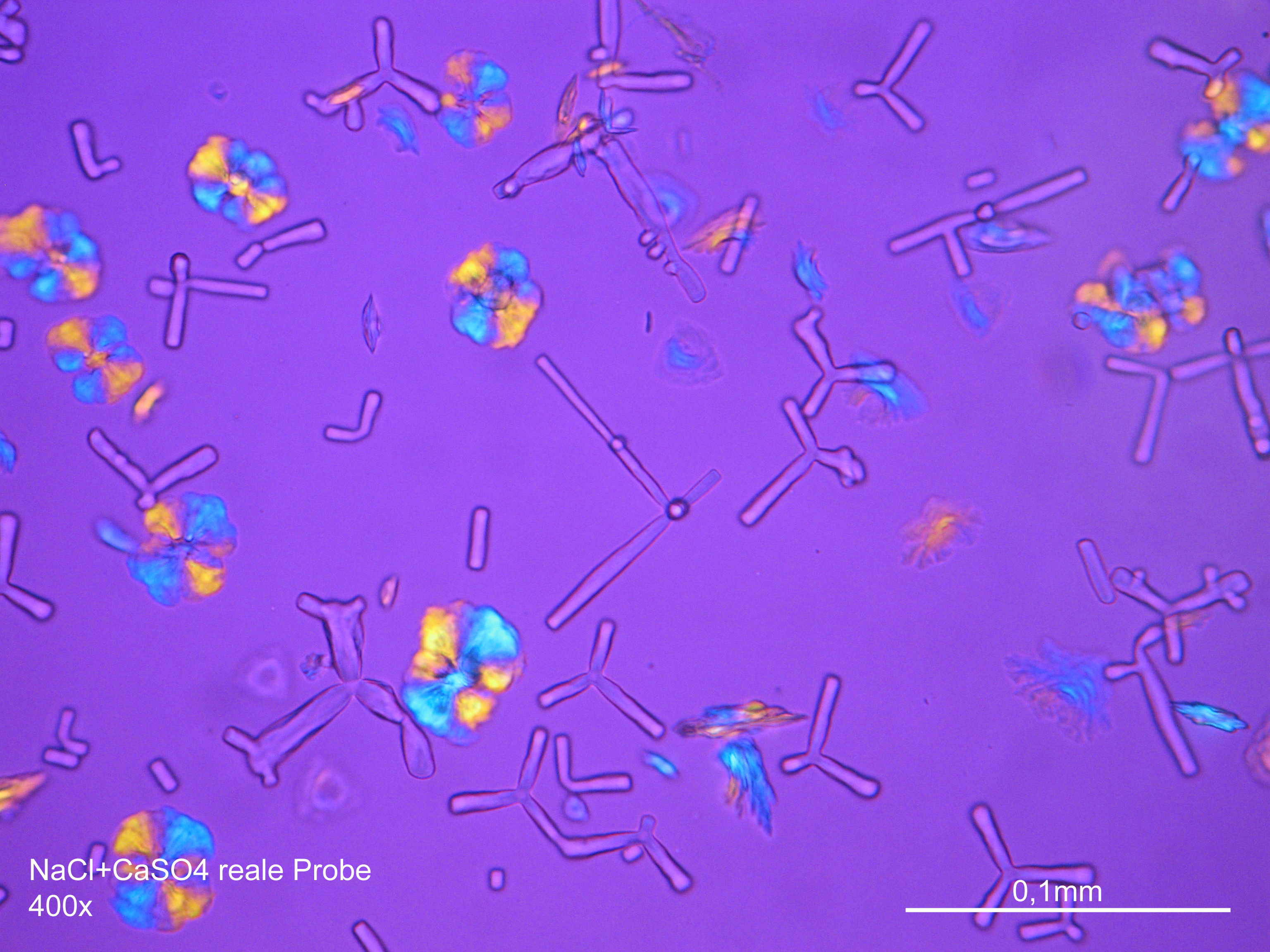
Image:NaCl+CaSO4 reale Probe 01.JPG|Sodium chloride and calcium sulphate in real sample, precipitate from watery solution on microscopic slide | |||
Image:NaCl+CaSO4 reale Probe 02.JPG|Sodium chloride and calcium sulphate in real sample, precipitate from watery solution on microscopic slide | |||
</gallery> | |||
<!-- | |||
=== Through the Scanning Electron Microscope === | |||
--> | |||
== Weblinks == | |||
<references/> | <references/> | ||
[[Category: | |||
== Literature == | |||
<!-- System NaCl-H2O was by BRAITSCH (1962) and FRENZEL (1980) --> | |||
<bibprint /> | |||
[[Category:Halit]] [[Category:HSchwarz]] [[Category:R-MSteiger]] [[Category:Bearbeitung]] [[Category:Chlorid]] [[Category:Salz]] | |||
Revision as of 08:54, 12 January 2012
<bibimport />
Autoren: Hans-Jürgen Schwarz, Nils Mainusch
English version by Christa Gerdwilker
zurück zu Chloride
| Halite[1][2] | |

| |
| Mineralogical name | Halite |
| Chemical name | Sodium chloride |
| Trivial name | Common Salt, Rock Salt |
| Chemical formula | NaCl |
| Other forms | Sodium chloride dihydrate/Hydrohalite (NaCl•2H2O)[3] |
| Crystal system | cubic |
| Crystal structure | |
| Deliquescence humidity 20°C | 75.7% (10°C), 75.3% (25°C) |
| Solubility (g/l) at 20°C | 358 g/l |
| Density (g/cm³) | 2.163 g/cm3 |
| Molar volume | 27.02 cm3/mol |
| Molar weight | 58.44 g/mol |
| Transparency | transparent to translucent |
| Cleavage | perfect |
| Crystal habit | cubic crystal, granular, massive aggregates |
| Twinning | none |
| Phase transition | |
| Chemical behavior | |
| Comments | slightly watersoluble |
| Crystal Optics | |
| Refractive Indices | n=1.544 |
| Birefringence | |
| Optical Orientation | isotropic |
| Pleochroism | |
| Dispersion | |
| Used Literature | |
| {{{Literature}}} | |
Abstract[edit]
Halite origins[edit]
Sodium chloride is obtained through mining or derived from the sea or salt lakes and is commonly used for cooking or as road gritting salt.
The salt content of sea water is approx. 2,7 M.%.
Causes of halite formation on monuments[edit]
Contact with materials containing soluble sodium-based ingredients can result in the efflorescence of sodium chloride on monuments. A primary example is the high sodium content of cements. Contamination with sodium and chloride ions can also occur through contact with salt laden ground or surface water. A range of cleaning materials (e.g. acidic and caustic cleaners) and especially previously used restoration materials (e.g. water glass) can introduce sodium and chloride ions into monuments. Further common sources of halite are rock salt used in road gritting which predominantly consists of sodium chloride, as well as salt laden seawater in coastal areas.
Solubility behavior[edit]
The commonly occurring halite found in northern Germany has a solubility of 358 g/l (20°C) and thus belongs to the group of very soluble and, therefore, easily mobilized salts. Its solubility changes comparatively little within a temperature range of 10 -30°C.
| Temperature | 10°C | 20°C | 40°C |
| Solubility [g/l] | 356,5 | 358,8 | 364,2 |
Hygroscopicity[edit]
Halite’s deliquescence humidity of approx. 75% is often crossed in the climate of northern Europe. Temperature variations hardly affect the deliquescence point of halite which is illustrated below in comparison with potassium nitrate and natrite.
Moisture sorption:
Theoretically 1g NaCl can absorb 4,3g moisture. The moisture sorption during varying relative humidity levels is:
| Relative humidity during storge/salt phase | NaCl |
| 87% RH | 153 |
| 81% RH | 22 |
| 79% RH | 7 |
Crystallization pressure[edit]
The crystallization of halite from a water-based solution results in a crystallization pressure of 55,4-65,4 N/mm2 [Winkler:1975]Title: Stone: Properties, Durability in Man´s Environment
Author: Winkler, Erhard M. (for comparison, the crystallization pressure of different salts can range from 7,2-65,4 N/mm2). These values need to be considered in conjunction with temperature and concentration and can, therefore, only act as indicators of damage potential in relation to salt crystallization pressure. In comparison to other salts, the crystallization pressure of halite is extremely high.
(for comparison, the crystallization pressure of different salts can range from 7,2-65,4 N/mm2). These values need to be considered in conjunction with temperature and concentration and can, therefore, only act as indicators of damage potential in relation to salt crystallization pressure. In comparison to other salts, the crystallization pressure of halite is extremely high.
Hydration behavior[edit]
Under normal conditions only the unhydrated form of halite exists. Only at a temperature of below 0.15 °C does a saturated water-based sodium chloride solution result in the precipitation of a deposit of dihydrate hydrohalite[4].
Microscopy[edit]
Laboratory examinations[edit]
Sodium chloride crystals can be reliably identified on the basis of morphological features. Individual particles usually form cubic or octahedral shapes and, therefore, clearly display right angles in their crystal construction.
Refractive index: nD = 1,544
Crystal category: cubic
Examinations under the polarising microscope:
Together with potassium chloride, sodium chloride is one of the few salts belonging to the cubic crystal system which cause damage to masonry. Because of its isotropic internal structure it does not display birefringence.
The classification of the refractive index occurs by immersion method in a standard oil with a refractive index of nD =1,518. Halite crystals display the same optical density in every direction so that the speed and orientation of linear polarised light waves are not distorted. When viewed between crossed polars, the crystals are not visible but appear (independent of orientation) extinguished.
Differentiating of halite from similar salts:
The group of isotropic salts causing masonry damage consists of halite, sylvine and fluorite. All these phases can be differenciated easily.
| Salt phase | Identification features |
| Sylvine KCl | Refractive index under 1,518. |
| Fluorite CaF2 | Refractive index under 1,518, barely water soluble. |
Images of salts and salt damage[edit]
On the object[edit]
Under the polarising microscope[edit]
- Precipitate from watery sample on microscope slide
Weblinks[edit]
- ↑ http://webmineral.com/data/Halite.shtml viewed on 28/07/2010
- ↑ http://www.mindat.org/min-1804.html viewed on 28/07/2010
- ↑ www.mindat.org/min-1804.html viewed on 28/07/2010
- ↑ http://www.mineralienatlas.de/lexikon/index.php/MineralData?mineral=Hydrohalit accessed 28.07.2010
Literature[edit]
[Filter missing]