Halite
Authors: Hans-Jürgen Schwarz, Nils Mainusch
English version by Christa Gerdwilker
back to Chloride
| Halite[1][2][3] | |

| |
| Mineralogical name | Halite |
| Chemical name | Sodium chloride |
| Trivial name | Common Salt, Rock Salt |
| Chemical formula | NaCl |
| Other forms | Sodium chloride dihydrate/Hydrohalite (NaCl•2H2O)[4] |
| Crystal system | cubic |
| Crystal structure | |
| Deliquescence humidity 20°C | 75.4% [Steiger etal: 2104]The entry doesn't exist yet. |
| Solubility (g/l) at 20°C | 358 g/l |
| Density (g/cm³) | 2.163 g/cm3 |
| Molar volume | 27.02 cm3/mol |
| Molar weight | 58.44 g/mol |
| Transparency | transparent to translucent |
| Cleavage | perfect |
| Crystal habit | cubic crystal, granular, massive aggregates |
| Twinning | none |
| Phase transition | |
| Chemical behavior | |
| Comments | water soluble |
| Crystal Optics | |
| Refractive Indices | n=1.544 |
| Birefringence | |
| Optical Orientation | isotropic |
| Pleochroism | |
| Dispersion | |
| Used Literature | |
| {{{Literature}}} | |
Abstract[edit]
Occurrence[edit]
Sodium chloride is obtained through mining or derived from the sea or salt lakes and is commonly used for cooking or as deicing salt for roads.
The sodium chloride content of sea water is around 2.7 M.%.
Origin of the halite found on monuments[edit]
Sodium chloride can enter buildings or monuments when these are in contact with materials containing this salt or even other salts containing either sodium or chloride, that might combine to produce NaCl in or efflorescence on them. Contamination with sodium and chloride ions can also occur through contact with salt laden ground or surface water. A range of cleaning materials (e.g., acidic and alkaline cleaners or combination of them), or previously used restoration materials (e.g., water glass) as well as Portland cement, can introduce sodium and chloride ions into monuments. Further common and important sources are deicing salts and maritime environments where the air and fogs may contain a significant amount of sodium chloride in suspension or dissolved in droplets.
Solubility behavior[edit]
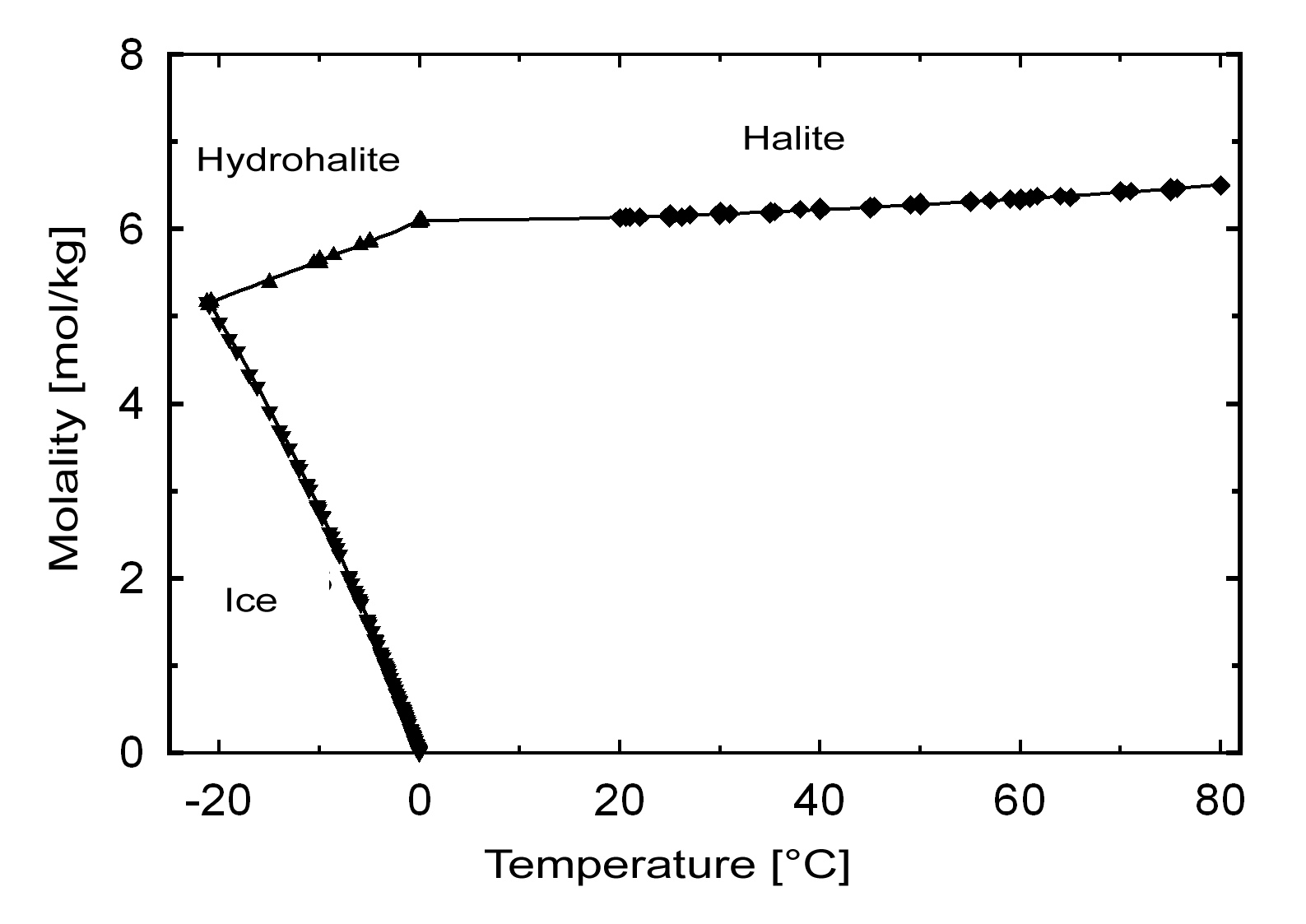
The commonly occurring halite has a solubility of 358 g/l (20°C) and can be considered a very soluble and, therefore, easily mobilized salt. Its solubility changes comparatively little within a temperature range of 10 -30°C.
| Temperature | 10°C | 20°C | 40°C |
| Solubility [g/l] | 356,5 | 358,8 | 364,2 |
Hygroscopicity[edit]
Halite has a deliquescence humidity of about 75% RH, therefore it tends to pick up moisture easily in most temperate climates.
| 0°C | 10°C | 20°C | 30°C | 40°C | 50°C |
| 75.9%r.h. | 75.6%r.h. | 75.4%r.h. | 75.2%r.h. | 75.2%r.h. | 74.8%r.h. |
Moisture sorption:
Theoretically 1g NaCl can take up 4.3g of moisture, i.e., water vapor. The moisture sorption during varying relative humidity levels is:
| Relative humidity during storge/salt phase | NaCl |
| 87% RH | 153 |
| 81% RH | 22 |
| 79% RH | 7 |
Hydration behavior[edit]
Under normal environmental conditions only halite will crystallize out of a saturated solution. The hydrated form, dihydrate hydrohalite[4] will only precipitate out at temperatures below 0.15°C.
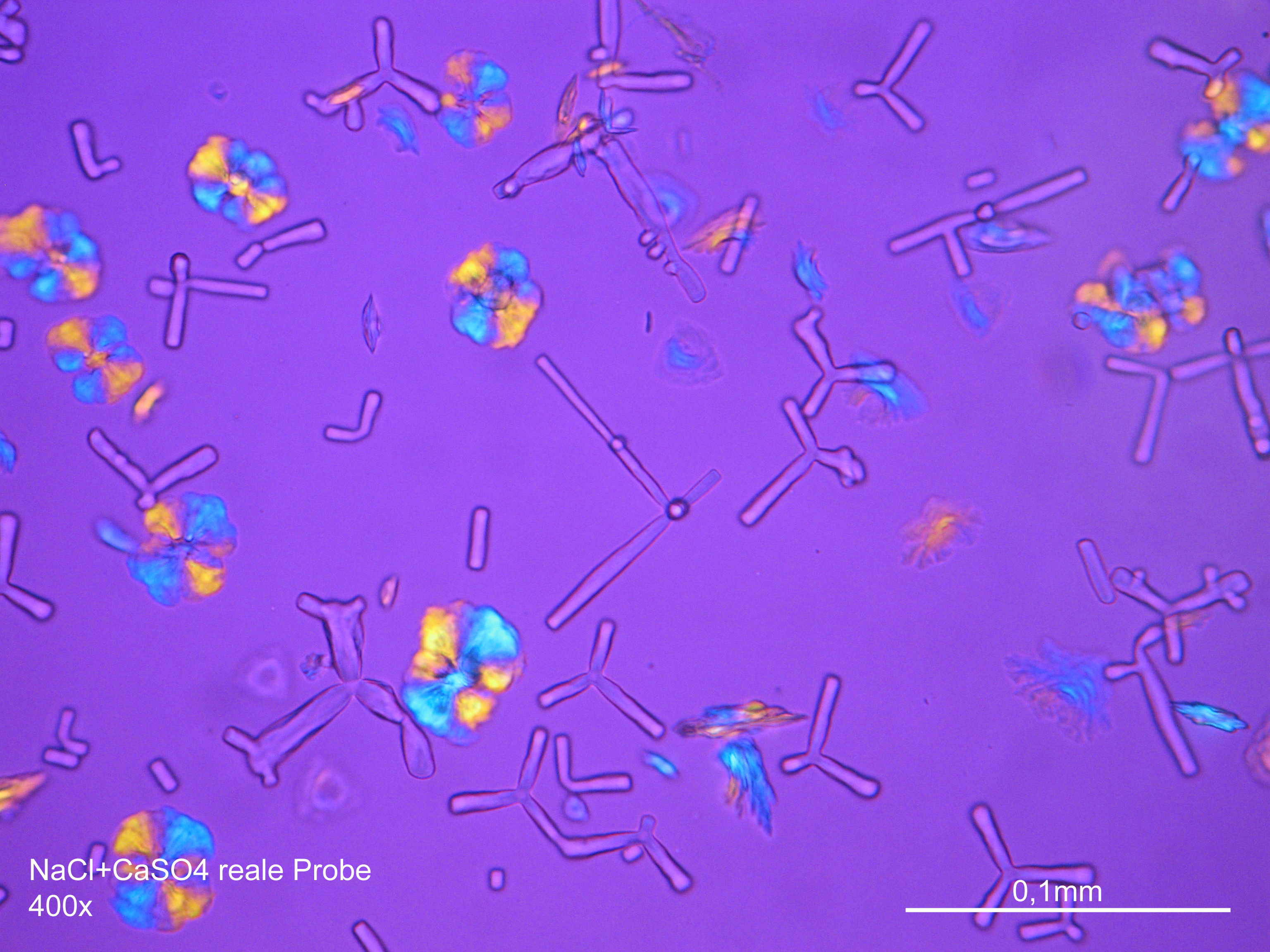
Microscopy[edit]
Laboratory analysis[edit]
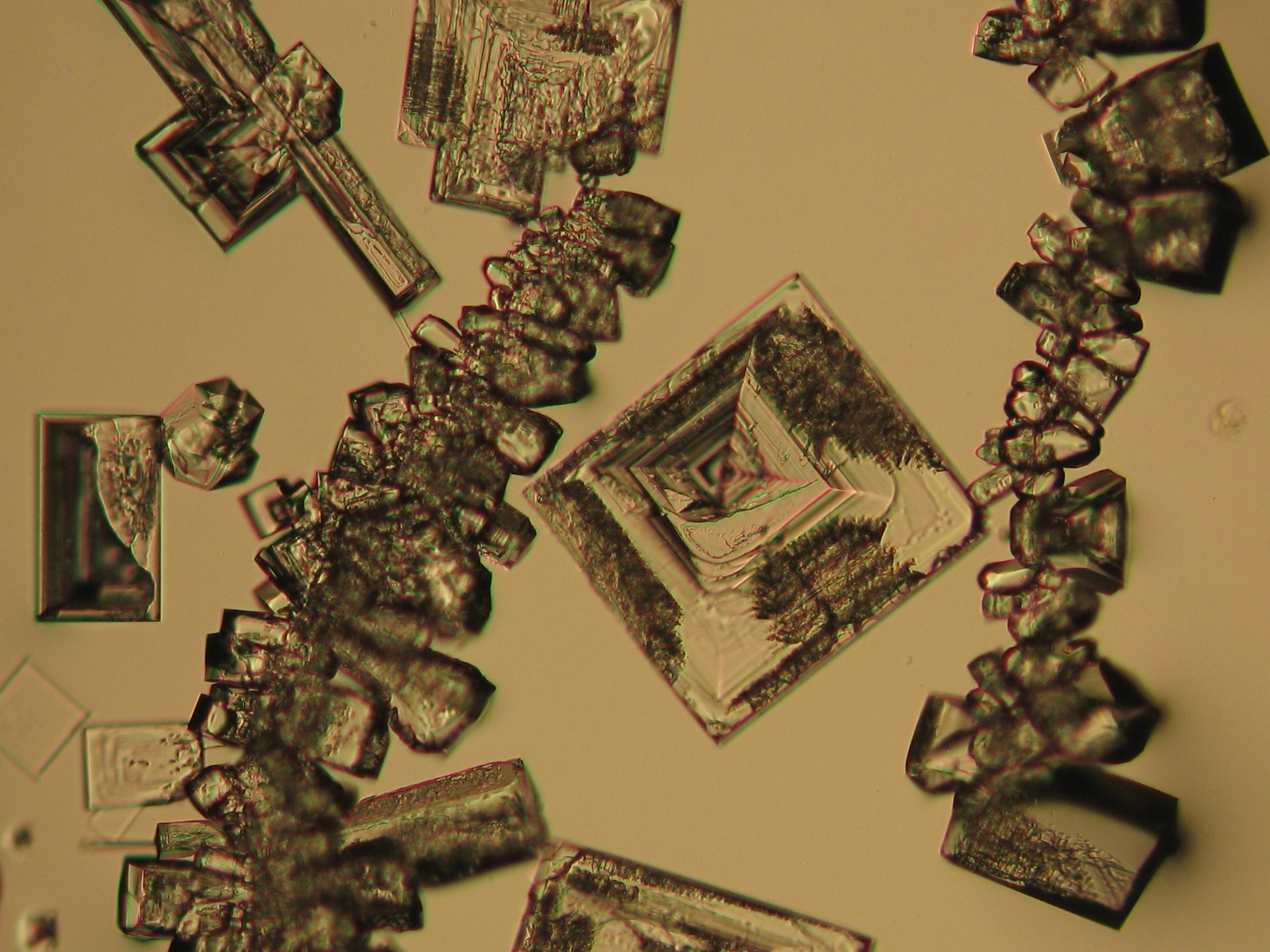
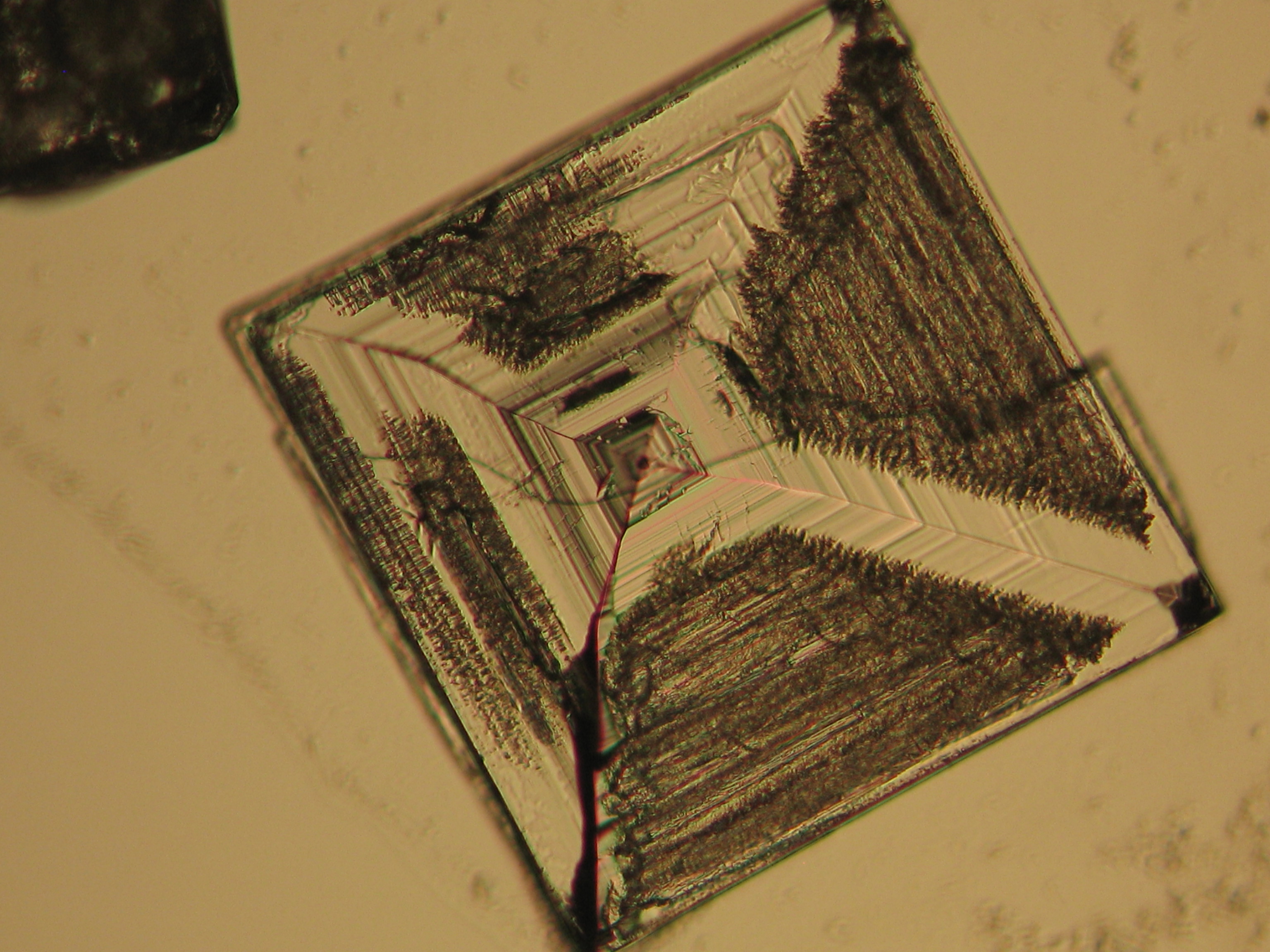
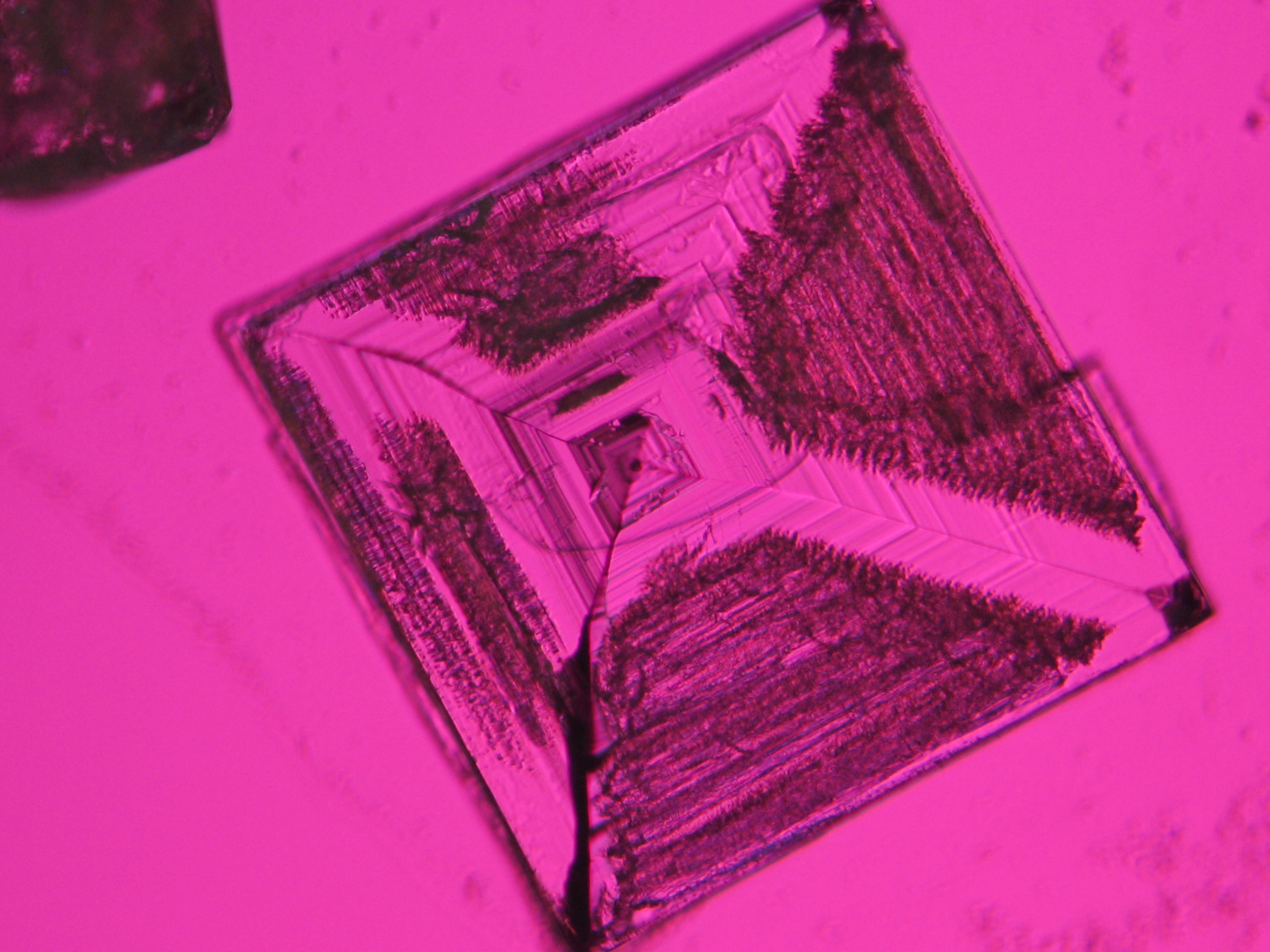
Sodium chloride crystals can be reliably identified on the basis of their morphological features. Individual particles usually form cubic or octahedral shapes and, therefore, clearly display right angles in their crystal construction.
Refractive index: nD = 1.544
Crystal category: cubic
Examination by polarized microscopy:
There are few salts belonging to the cubic crystal system which can be found in masonry, i.e., sodium chloride (halite), potassium chloride (sylvite) and calcium fluoride (flourite). Only the two first salts are highly soluble and therefore they are the ones that can cause damage to the masonry. Because of its isotropic internal structure these salts do not display birefringence.
The refractive index can be measured with the immersion method by using a standard oil with a refractive index of nD =1.518. Halite crystals display the same optical density in every direction so that the speed and orientation of linear polarized light waves are not distorted, therefore, when viewed between crossed polars, the crystals are not visible, i.e., they show "extinction".
Differentiation of halite from similar salts:
The three above mentioned isotropic salts can be easily differentiated.
| Salt phase | Identification features |
| Sylvine KCl | Refractive index below 1,518. |
| Fluorite CaF2 | Refractive index below 1,518, barely water soluble. |
Images of salts and salt damage[edit]
In situ[edit]
Under the polarizing microscope[edit]
- Precipitated from the evaporation of an aqueous solution of the sample on microscope slides
Scanning Electron Microscope photomicrographs[edit]
Weblinks[edit]
- ↑ http://webmineral.com/data/Halite.shtml accessed 28.07.2010
- ↑ http://www.mindat.org/min-1804.html accessed 28.07.2010
- ↑ http://www.mineralienatlas.de/lexikon/index.php/MineralData?mineral=Halit accessed 28.07.2010
- ↑ 4.0 4.1 http://www.mineralienatlas.de/lexikon/index.php/MineralData?mineral=Hydrohalit accessed 28.07.2010
Literature[edit]
| [Dana:1951] | Dana E.S. (eds.) Dana J.D. (1951): Dana's System of Mineralogy, 7, Wiley & Sons |  |
| [Robie.etal:1978] | Robie R.A., Hemingway B.S.; Fisher J.A. (1978): Thermodynamic properties of minerals and related substances at 298.15 K and 1 bar pressure and higher temperatures. In: U.S. Geol. Surv. Bull, 1452 () |  |
| [Steiger] | The entry doesn't exist yet. | |
| [Steiger.etal:2008c] | Steiger, Michael; Kiekbusch, Jana; Nicolai, Andreas (2008): An improved model incorporating Pitzer’s equations for calculation of thermodynamic properties of pore solutions implemented into an efficient program code. In: Construction and Building Materials, 22 (8), 1841-1850, 10.1016/j.conbuildmat.2007.04.020 |  |
| [Steiger.etal:2014] | Steiger, Michael; Charola A. Elena; Sterflinger, Katja (2014): Weathering and Deterioration. In: Siegesmund S.; Snethlage R. (eds.): Stone in Architecture, Springer Verlag Berlin Heidelberg, 223-316, 10.1007/978-3-642-45155-3_4. |  |
| [Vogt.etal:1993] | Vogt, R.; Goretzki, Lothar (1993): Der Einfluss hygroskopischer Salze auf die Gleichgewichtsfeuchte und Trocknung anorganischer Baustoffe, unveröffentlichter Bericht. |  |
| [Winkler:1975] | Winkler, Erhard M. (1975): Stone: Properties, Durability in Man´s Environment, Springer Verlag, Wien |  |
More Literature
| [Biscontin.etal:1988] | Biscontin, G.; Driussi, G.; Masin, A.; Zendri, E. (1988): Study on stones artificially salted with MgSO4*7H2O and NaCl and treated with siliconic resin. In: Ciabach, J. (eds.): Proceedings of the 6th International Congress on Deterioration and Conservation of Stone,Nicholas Copernicus University 194-206. |  |
| [Brown.etal:2000] | Brown, P. W.; Badger, S. (2000): The distributions of bound sulfates and chlorides in concrete subjected to mixed NaCl, MgSO4, Na2SO4 attack. In: Cem. Concr. Res., 30 (10), 1535-1542 |  |
| [Brown.etal:2001] | Brown, P. W.; Badger, S. (2001): Reply to the discussion by William G. Hime and Stella L. Marusin of the paper "The distribution of bound sulfates and chlorides in concrete to mixed NaCl, MgSO4, Na2SO4 attack". In: Cem. Concr. Res., 31 (7), 1117-1118 |  |
| [Dorn.etal:2007] | Dorn, Joachim; Steiger, Michael (2007): Measurement and Calculation of Solubilities in the Ternary System NaCH3COO + NaCl + H2O from 278 K to 323 K. In: Journal of Chemical and Engineering Data, 5 (52), 1784-1790, 10.1021/je7001495 |  |
| [Friedel:1978] | Friedel, B. (1978): Gipslöslichkeiten in wässerigen Systemen mit NaCl, MgCl2, Na2SO4 und MgSO4. In: Zeitschrift für Pflanzenernährung und Bodenkunde, 141 (3), 337-346, 10.1002/jpln.19781410309 |  |
| [Glasner.etal:1974] | Glasner, A.; Zidon, M. (1974): The crystallization of NaCl in the presence of (Fe(CN)6)4- ions. In: Journal of Crystal Growth, 21 (2), 294-304, 10.1016/0022-0248(74)90018-9 |  |
| [Linnow.etal:2007c] | Linnow, Kirsten; Juling, Herbert; Steiger, Michael (2007): Investigation of NaCl deliquescence in porous substrates using RH-XRD. In: Environmental Geology, 52 (2), 317-327, 10.1007/s00254-006-0590-9 |  |
| [Lubelli.etal:2006] | Lubelli, B.; van Hees, R.P.J.; Huinik, H.P.; Groot, C.J.W.P. (2006): Irreversible dilation of NaCl contaminated lime–cement mortar due to crystallization cycles. In: Cement and Concrete Research, 36 (4), 678-687, 10.1016/j.cemconres.2005.10.008 |  |
| [Lubelli.etal:2006a] | Lubelli, B.; van Hees, R.P.J.; Huinink, H.P. (2006): Effect of NaCl on the hydric and hygric dilation behaviour of lime-cement mortar. In: HERON, 51 (1), 33-48 |   |
| [Marliacy.etal:2000] | Marliacy, P.; Solimando, R.; Bouroukba, M.; Schuffenecker, L. (2000): Thermodynamics of crystallization of sodium sulfate decahydrate in H2O-NaCl-Na2SO4: application to Na2SO4.cntdot.10H2O-based latent heat storage materials. In: Thermochim. Acta, 344 (1), 85-94 |  |
| [Monnin:1990] | Monnin, C. (1990): The influence of pressure on the activity coefficients of the solutes and on the solubility of minerals in the system Na-Ca-Cl-SO4-H2O to 200°C and 1 kbar, and to high NaCl concentration. In: Geochimica et Cosmochimica Acta, 54 (12), 3265-3282, 10.1016/0016-7037(90)90284-R |  |
| [Moropoulou.etal:1992] | Moropoulou, Antonia; Theoulakis, Panagiotis (1992): Conditions causing destructive NaCl crystallization into the porous sandstone, building material of the medieval city of Rhodes. In: Decrouez, Danielle; Chamay, Jacques; Zezza, Fulvio (eds.): The conservation of monuments in the Mediterranean Basin: proceedings of the 2nd international symposium, Musee d'Art et d'Histoire-Geneve; Museum d'Histoire Naturelle, 493-499. |  |
| [Ottosen.etal:2017] | Ottosen, Lisbeth M.; Andersson, Lovisa C. H. (2017): Electrode placement during electro-desalination of NaCl contaminated sandstone – simulating treatment of carved stones. In: Laue, Steffen (eds.): Proceedings of SWBSS 2017. Fourth International Conference on Salt Weathering of Buildings and Stone Sculptures, University of Applied Sciences Potsdam, Germany, 20-22 September 2017,Verlag der Fachhochschule Potsdam 150-157, 10.5165/hawk-hhg/332. |  |
| [Pitzer:1986] | Pitzer, K. S. (1986): Thermodynamics of NaCl in steam. In: Geochemica et Cosmochimica Acta, 50 (7), 1445-1454 |  |
| [Platford:1975] | Platford, R. F. (1975): Thermodynamics of the system H2O-NaCl-MgCl2-Na2SO4-MgSO4 at 25 degrees C. In: Mar. Chem., 3 (4), 261-270 |  |
| [Potter.etal:1978] | Potter, R. W. I.; Clynne, M. A. (1978): Solubility of high soluble salts in aqueous media; Part 1, NaCl, KCl, CaCl2, Na2SO4, and K2SO4 solubilities to 100 degrees C. In: Journal of Research of the U. S. Geological Survey, 6 (6), 701-705 |  |
| [Ptacek.etal:1992] | Ptacek, C. J.; Reardon, E. J. (1992): Solubility of siderite (FeCO3) in concentrated NaCl and Na2SO4 solutions at 25 degrees C. In: Kharaka, Yousif K.; Maest, Ann S. (eds.): Proceedings of the 7th international symposium on water-rock interaction, 181-184. |  |
| [Rodriguez-Navarro.etal:2002] | Rodriguez-Navarro, Carlos; Linares-Fernandez, Lucia; Doehne, Eric; Sebastian, Eduardo (2002): Effects of ferrocyanide ions on NaCl crystallization in porous stone. In: Journal of Crystal Growth, 243 (3), 503-516, Url |  |
| [Sarada.etal:1990] | Sarada, S.; Ananthaswamy, J. (1990): Thermodynamic Properties of Electrolyte Solutions: Emf Study of the System NaCl-Na2SO4-H20 at 25, 35 and 45 ÄC. In: Journal Chem. Soc. Faraday Trans., 86 (1), 81-84 |  |
| [Shichiri.etal:1965] | Shichiri, T.; Kato, N. (1965): The growth and dissolution of NaCl whiskers in aqueous solution. In: Acta Metallurg., 13 (), 785-795 |  |
| [Shichiri.etal:1967] | Shichiri, T.; Kinoshita, H.; Kato, N. (1967): Regrowth of NaCl and KCl whiskers in aqueous solution. In: Peiser, H.S. (eds.): Crystal Growth, , 385-388. |  |
| [Shichiri.etal:1968] | Shichiri, T.; Kato, N. (1968): Regrowth of NaCl whiskers from pure and poisoned solutions. In: Journal of Crystal Growth, 3/4 (), 384-390, Url |  |
| [Simon:1981] | Simon, B. (1981): Dissolution rates of NaCl and KCl in auqeous solution. In: Journal of Crystal Growth, 52 (2), 789-794, Url |  |
| [Simon:1983] | (1983): Influence of the direction of the solution flow on the morphology of NaClO3 crystals. In: Journal of Crystal Growth, 61 (1), 167-169, Url |  |
| [Sonnleitner:2011] | Sonnleitner,Tobias (2011): Symmetrien und Manipulation des Ladungszustands von Molekülen auf NaCl-Filmen, Url. |  |
| [Tishchenko.etal:1992] | Tishchenko, Pavel Ya.; Bychkov, Alexander S.; Hravéczy-Páll, Andrea; Tóth, Klára; Pungor, Ernoe (1992): Activity Coefficients for the System NaCl + Na2SO4 + H2O at Various Temperatures. Application of Pitzer's Equations. In: Journal of Solution Chemistry, 21 (3), 261-274 |  |
| [Vazquez.etal:2014] | Vázquez, P.; Thomachot-Schneider, C.; Mouhoubi, K.; Gommeaux, M.; Fronteau, G.; Barbin, V.; Bodnar, J.L. (2014): Study of NaCl crystallization using Infrared Thermography. In: Hilde De Clercq (eds.): Proceedings of SWBSS 2014 3rd International Conference on Salt Weathering of Buildings and Stone Sculptures,KIK-IRPA, Royal Institute for Cultural Heritage Brussels 289-303, 10.5165/hawk-hhg/274. |   |
| [Wolf.etal:1992] | Wolf, Manfred; Rohde, Harald (1992): Solubility of calcite in mixed aqueous solutions of NaCl and KCl at 25 degrees C and CO2 partial pressures of about 1 kPa. In: Proceedings of the 7th international symposium on water rock interaction, 7 (), 195 |  |
| [Yellin.etal:1985] | Yellin, N.; Zelingher, N.; Ben-Dor, L. (1985): Whiskers growth by means of cellulose acetate membranes: NaCl and KCl. In: Journal of Crystal Growth, 71 (2), 427-432, Url |  |
| [Zdanovskii.etal:1991] | Zdanovskii, A. B.; Frolovskii, E. E. (1991): Equations for calculating the solubility of mirabilite in the aqueoussodium chloride-magnesium sulfate (2NaCl + MgSO4 = Na2SO4 + MgCl2) system at 0-25.degree. In: Zh. Prikl. Khim. (Leningrad), 64 (6), 1153-7 |  |
| [Zelingher:1975] | Zelingher, N. (1975): A new method of growing NaCl and KCl whiskers. In: Acta Crystallogr., A 31 Suppl., (), 211 |  |