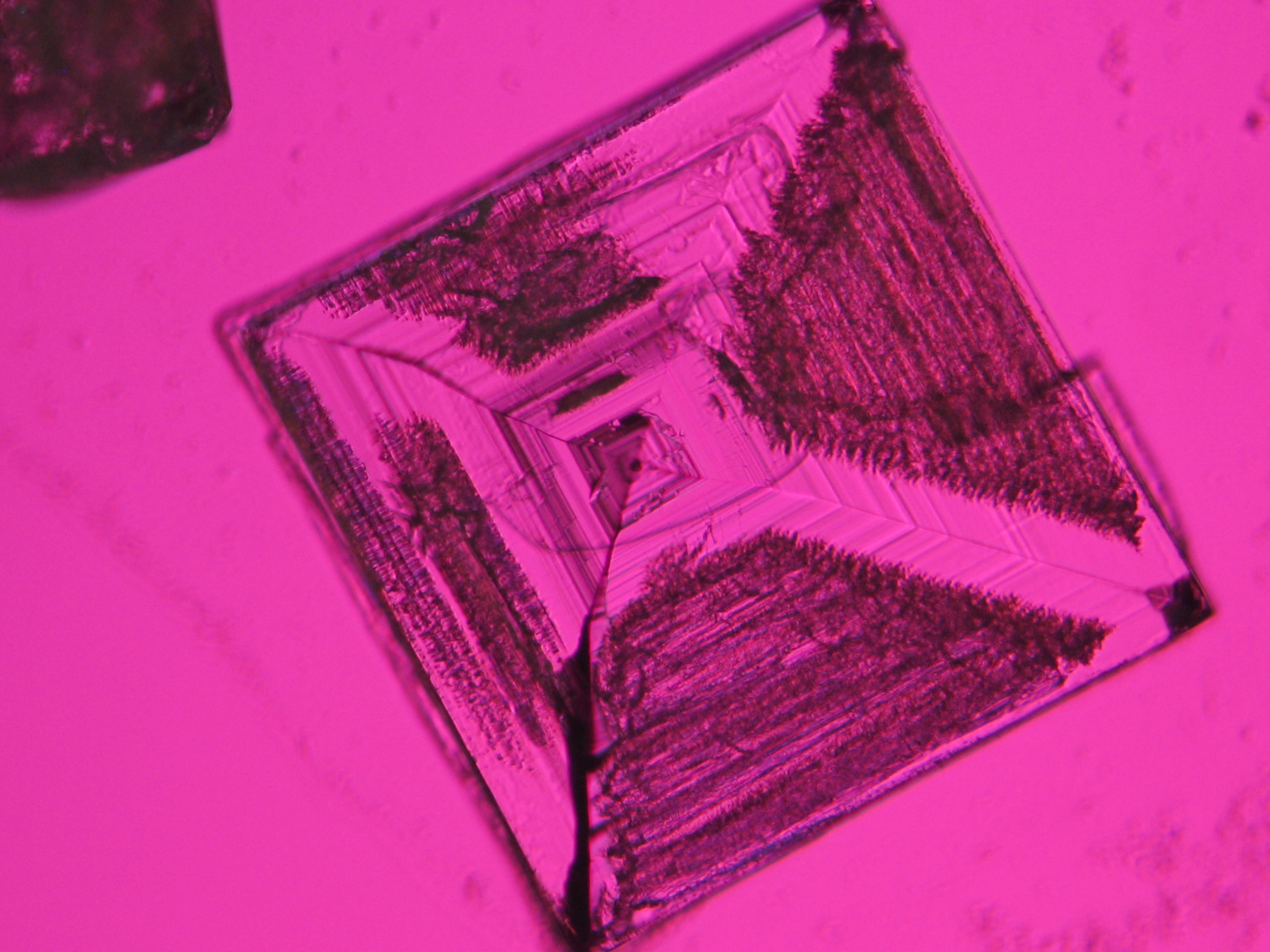
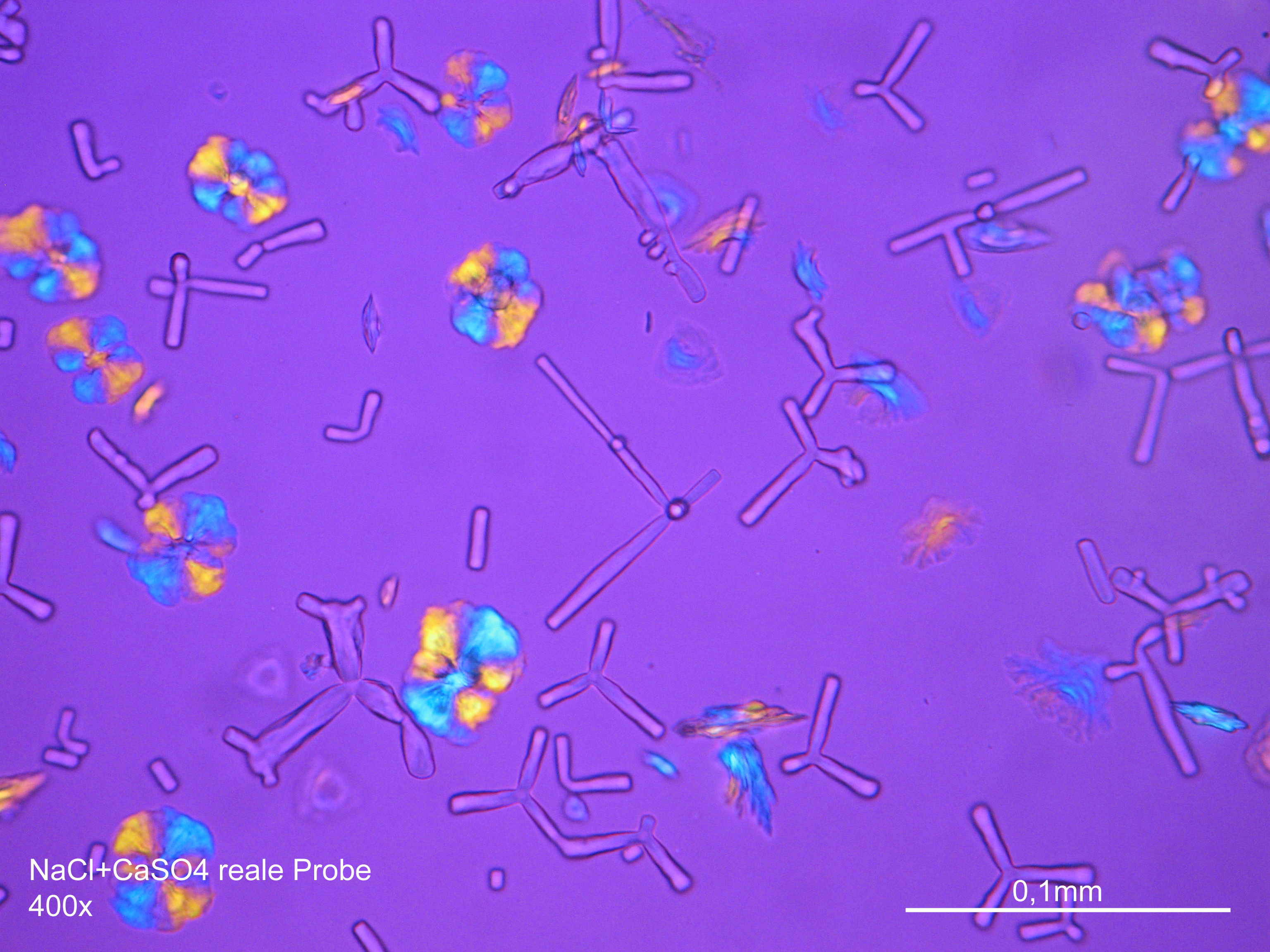
Halite
Authors: Hans-Jürgen Schwarz, Nils Mainusch
English version by Christa Gerdwilker
back to Chloride
| Halite[1][2][3] | |

| |
| Mineralogical name | Halite |
| Chemical name | Sodium chloride |
| Trivial name | Common Salt, Rock Salt |
| Chemical formula | NaCl |
| Other forms | Sodiumchloride Dihydrate/Hydrohalite (NaCl•2H2O)[4] |
| Crystal system | cubic |
| Crystal structure | |
| Deliquescence humidity 20°C | 75.7% (10°C), 75.3% (25°C) |
| Solubility (g/l) at 20°C | 358 g/l |
| Density (g/cm³) | 2.163 g/cm3 |
| Molar volume | 27.02 cm3/mol |
| Molar weight | 58.44 g/mol |
| Transparency | transparent to translucent |
| Cleavage | perfect |
| Crystal habit | cubic crystal, granular, massive aggregates |
| Twinning | none |
| Phase transition | |
| Chemical behavior | |
| Comments | water soluble |
| Crystal Optics | |
| Refractive Indices | n=1.544 |
| Birefringence | |
| Optical Orientation | isotropic |
| Pleochroism | |
| Dispersion | |
| Used Literature | |
| {{{Literature}}} | |
Abstract[edit]
Occurrence[edit]
Sodium chloride is obtained through mining or derived from the sea or salt lakes and is commonly used for cooking or as deicing salt for roads.
The salt content of sea water is around 2.7 M.%.
Information on the origins and formation of halite on monuments[edit]
Sodium chloride can enter buildings or monuments when these are in contact with materials containing this salt or even other salts containing either sodium or chloride, that might react to produce NaCl efflorescence on them. Contamination with sodium and chloride ions can also occur through contact with salt laden ground or surface water. A range of cleaning materials (e.g., acidic and alkaline cleaners) and especially previously used restoration materials (e.g., water glass) or even Portland cement, can introduce sodium and chloride ions into monuments. Further common and important sources are deicing salts and maritime environments where the air and fogs may contain a significant amount of sodium chloride in suspension or dissolved in droplets.
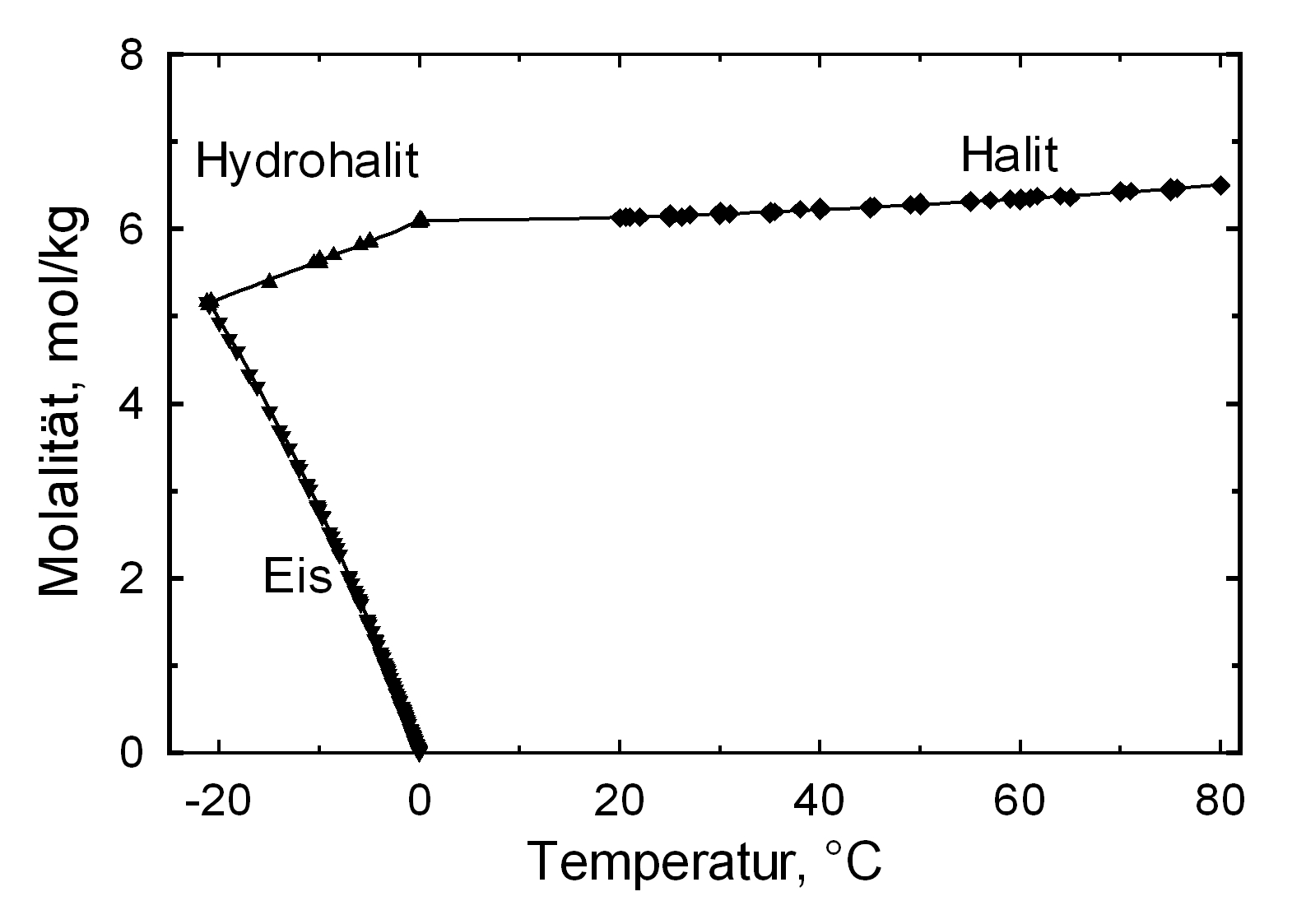
Solubility behavior[edit]
The commonly occurring halite found in northern Germany has a solubility of 358 g/l (20°C) and thus belongs to the group of very soluble and, therefore, easily mobilized salts. Its solubility changes comparatively little within a temperature range of 10 -30°C.
| Temperature | 10°C | 20°C | 40°C |
| Solubility [g/l] | 356,5 | 358,8 | 364,2 |
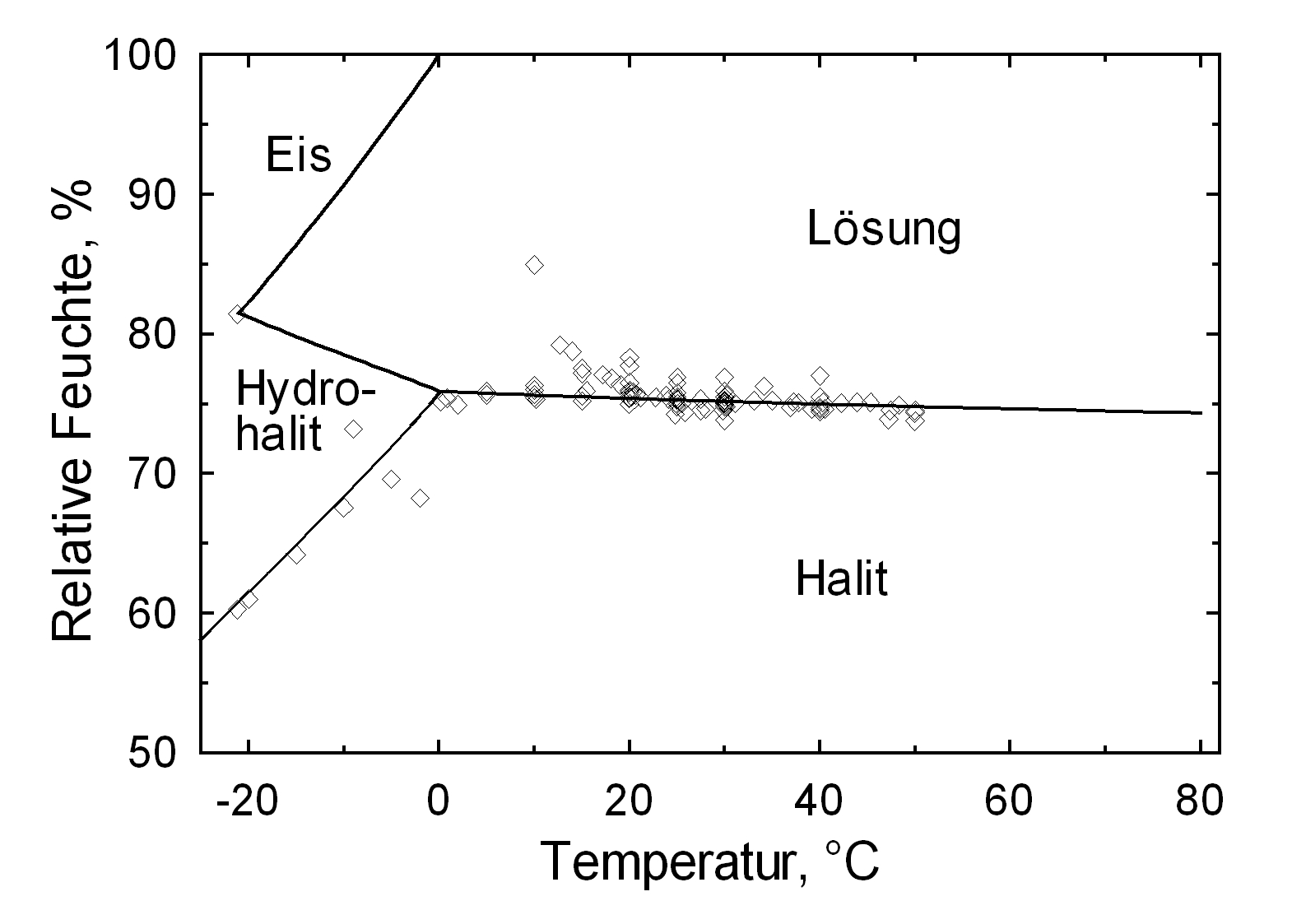
Hygroscopicity[edit]
Halite’s deliquescence humidity of approx. 75% RH is often crossed in the climate of northern Europe.
Moisture sorption:
Theoretically 1g NaCl can absorb 4,3g moisture. The moisture sorption during varying relative humidity levels is:
| Relative humidity during storge/salt phase | NaCl |
| 87% RH | 153 |
| 81% RH | 22 |
| 79% RH | 7 |
Crystallization pressure[edit]
The crystallization of halite from an aqueous solution results in a crystallization pressure of 55,4-65,4 N/mm2 [Winkler:1975]Title: Stone: Properties, Durability in Man´s Environment
Author: Winkler, Erhard M. (for comparison, the crystallization pressure of different salts can range from 7,2-65,4 N/mm2). These values need to be considered in conjunction with temperature and concentration and can, therefore, only act as indicators of damage potential in relation to salt crystallization pressure. In comparison to other salts, the crystallization pressure of halite is extremely high.
(for comparison, the crystallization pressure of different salts can range from 7,2-65,4 N/mm2). These values need to be considered in conjunction with temperature and concentration and can, therefore, only act as indicators of damage potential in relation to salt crystallization pressure. In comparison to other salts, the crystallization pressure of halite is extremely high.
Hydration behavior[edit]
Under normal conditions only the unhydrated form of halite exists. Only at a temperature of below 0.15 °C does a saturated water-based sodium chloride solution result in the precipitation of a deposit of dihydrate hydrohalite[4].
Microscopy[edit]
Laboratory analysis[edit]
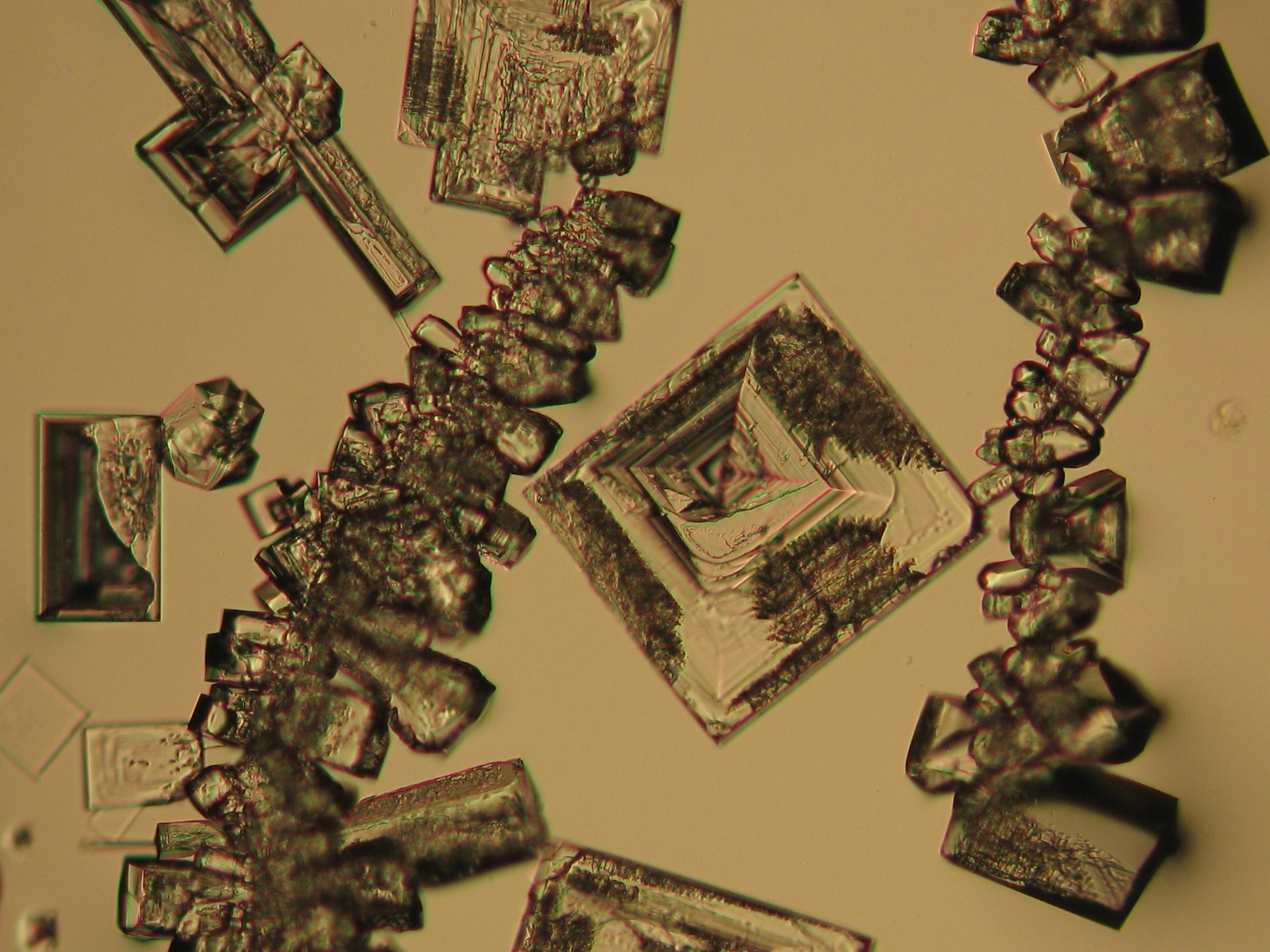
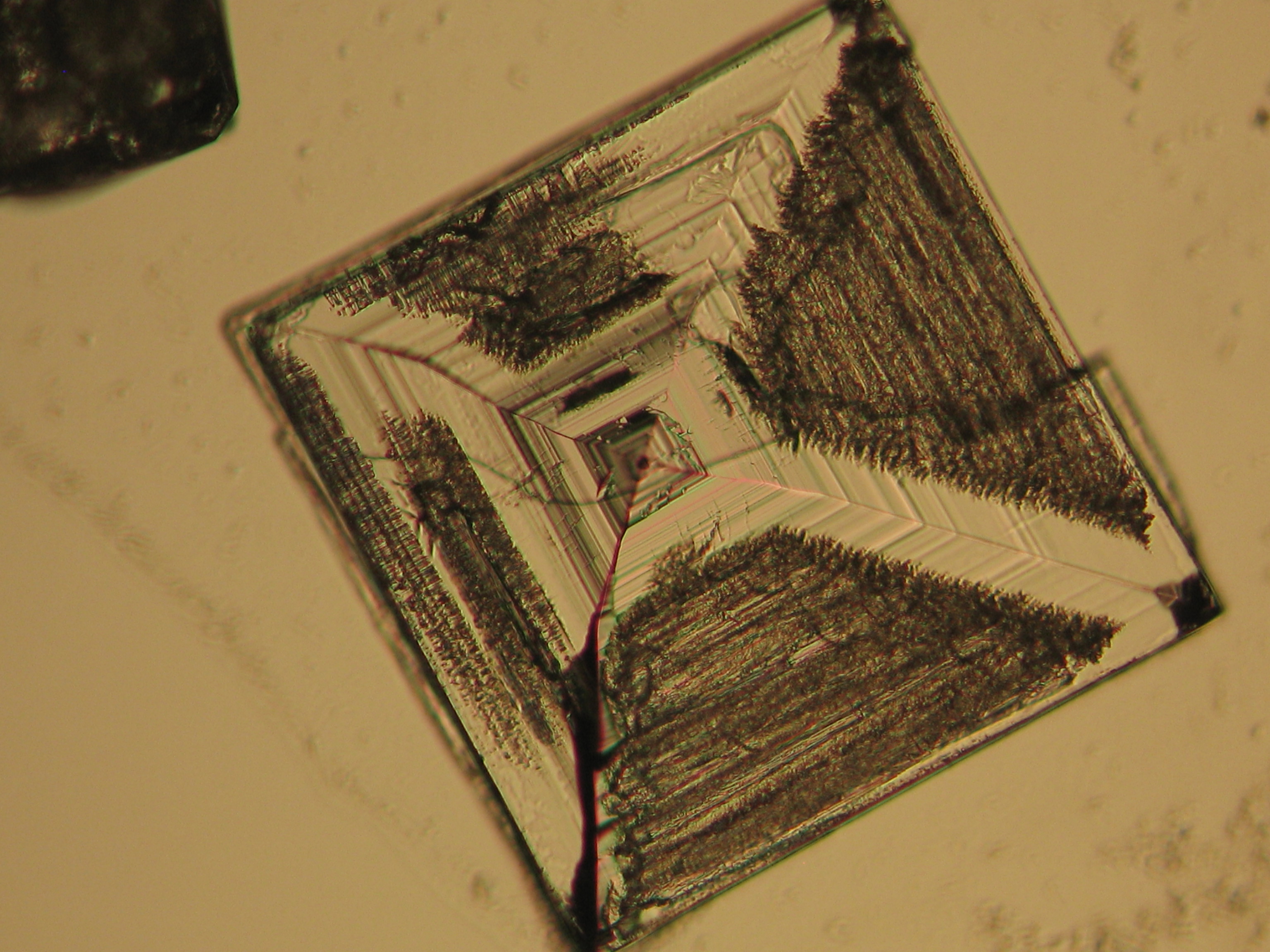
Sodium chloride crystals can be reliably identified on the basis of morphological features. Individual particles usually form cubic or octahedral shapes and, therefore, clearly display right angles in their crystal construction.
Refractive index: nD = 1.544
Crystal category: cubic
Examination by polarized microscopy:
Together with potassium chloride, sodium chloride is one of the few salts belonging to the cubic crystal system which cause damage to masonry. Because of its isotropic internal structure it does not display birefringence.
The classification of the refractive index occurs by immersion method in a standard oil with a refractive index of nD =1.518. Halite crystals display the same optical density in every direction so that the speed and orientation of linear polarized light waves are not distorted. When viewed between crossed polars, the crystals are not visible but appear (independent of orientation) extinguished.
Differentiation of halite from similar salts:
The group of isotropic salts causing masonry damage consists of halite, sylvite and fluorite. All these phases can be differentiated easily.
| Salt phase | Identification features |
| Sylvine KCl | Refractive index below1,518. |
| Fluorite CaF2 | Refractive index below 1,518, barely water soluble. |
Images of salts and salt damage[edit]
In situ[edit]
Under the polarizing microscope[edit]
- Precipitate from watery sample on microscope slide
Weblinks[edit]
- ↑ http://webmineral.com/data/Halite.shtml accessed 28.07.2010
- ↑ http://www.mindat.org/min-1804.html accessed 28.07.2010
- ↑ http://www.mineralienatlas.de/lexikon/index.php/MineralData?mineral=Halit accessed 28.07.2010
- ↑ Jump up to: 4.0 4.1 http://www.mineralienatlas.de/lexikon/index.php/MineralData?mineral=Hydrohalit accessed 28.07.2010
Literature[edit]
| [Dana:1951] | Dana E.S. (eds.) Dana J.D. (1951): Dana's System of Mineralogy, 7, Wiley & Sons |  |
| [Robie.etal:1978] | Robie R.A., Hemingway B.S.; Fisher J.A. (1978): Thermodynamic properties of minerals and related substances at 298.15 K and 1 bar pressure and higher temperatures. In: U.S. Geol. Surv. Bull, 1452 () |  |
| [Steiger] | The entry doesn't exist yet. | |
| [Steiger.etal:2008c] | Steiger, Michael; Kiekbusch, Jana; Nicolai, Andreas (2008): An improved model incorporating Pitzer’s equations for calculation of thermodynamic properties of pore solutions implemented into an efficient program code. In: Construction and Building Materials, 22 (8), 1841-1850, 10.1016/j.conbuildmat.2007.04.020 |  |
| [Steiger.etal:2014] | Steiger, Michael; Charola A. Elena; Sterflinger, Katja (2014): Weathering and Deterioration. In: Siegesmund S.; Snethlage R. (eds.): Stone in Architecture, Springer Verlag Berlin Heidelberg, 223-316, 10.1007/978-3-642-45155-3_4. |  |
| [Vogt.etal:1993] | Vogt, R.; Goretzki, Lothar (1993): Der Einfluss hygroskopischer Salze auf die Gleichgewichtsfeuchte und Trocknung anorganischer Baustoffe, unveröffentlichter Bericht. |  |
| [Winkler:1975] | Winkler, Erhard M. (1975): Stone: Properties, Durability in Man´s Environment, Springer Verlag, Wien |  |