Natrite
Authors: Hans-Jürgen Schwarz, Nils Mainusch
English version by Christa Gerdwilker
back to Carbonate
| Natrite[1][2] | |
| |
| Mineralogical name | Natrite |
| Chemical name | Sodium carbonate |
| Trivial name | |
| Chemical formula | Na2CO3 |
| Other forms | Thermonatrite (Na2CO3•H2O) Sodium carbonate heptahydrate (Na2CO3•7H2O) Natron (Na2CO3•10H2O) |
| Crystal system | monoclinic |
| Crystal structure | |
| Deliquescence humidity 20°C | |
| Solubility (g/l) at 20°C | 210 g/l |
| Density (g/cm³) | 2.532 g/cm3 |
| Molar volume | |
| Molar weight | 105.99 g/mol |
| Transparency | |
| Cleavage | good |
| Crystal habit | |
| Twinning | |
| Phase transition | |
| Chemical behavior | alkaline in aqueous solution, pH ≈ 12 |
| Comments | blooms quickly in dry air and passes into the monohydrate pH about 12 |
| Crystal Optics | |
| Refractive Indices | nx = 1.415 ny = 1.535 nz = 1.546 |
| Birefringence | Δ = 0.131 |
| Optical Orientation | biaxial negative |
| Pleochroism | |
| Dispersion | |
| Used Literature | |
Occurrence[edit]
Hydrated sodium carbonates occur naturally in solid salt deposits, in salt lakes, e.g., Lake Magadi in East Africa or Owens Lake in California, U.S.A. Varying amounts of natural sodium carbonate can also be found in alkaline springs. Different extraction techniques have been developed to produce pure sodium carbonate through dissolution, purification and evaporation processes.
Information on the origins and formation of natrite on monuments[edit]
Sodium carbonate can crystallize within the porous building materials when soluble sodium compounds in an alkaline environment, as explained below, react with the CO2 from the air leading to its eventual efflorescence.
Cements containing up to 0.5% soluble alkalis (in accordance with DIN standards) have a high content of sodium ions. It can be calculated that 1g of Na2O can lead to the formation of 4.6g of sodium carbonate decahydrate. If, for example, a Portland cement only contains 0.1% Na2O then 0.45kg of sodium carbonate can be formed per100kg of cement [Arnold.etal:1991]Title: Monitoring Wall Paintings Affected by soluble Salts
Author: Arnold, Andreas; Zehnder, Konrad . A large number of cleaning materials and, in particular, previously used conservation products (such as waterglass) contain sodium ions that remain in the building materials. Further sources are ground and surface water which may contain Na+-ions. Deicing salts may contain sodium chloride, which is very soluble. This salt is naturally present in coastal areas because of the seawater and NaCl may be found suspended in the fog and sea spray.
. A large number of cleaning materials and, in particular, previously used conservation products (such as waterglass) contain sodium ions that remain in the building materials. Further sources are ground and surface water which may contain Na+-ions. Deicing salts may contain sodium chloride, which is very soluble. This salt is naturally present in coastal areas because of the seawater and NaCl may be found suspended in the fog and sea spray.
Decay mechanisms of natrite[edit]
Solubility behavior[edit]
Natron, sodium carbonate decahydrate, is most commonly found as efflorescences on building materials. It can also crystallize as thermonatrite, the monohydrate, but it is unlikely to find the anhydrous salt, usually referred to as washing soda or soda ash. These salt(s) are very water soluble which and hence have high mobility within the porous network of the building material. Temperature has a pronounced influence on the solubility behavior of thermonatrite (the monohydrate) and soda ash (the anhydrous f0rm) in water. As a consequence, lowering of the temperature may result in the supersaturation of their water solutions with the subsequent precipitation of these salts, even though the water content remains the same. It also can form an metastable heptahydrate.
Diagram 1 – Illustration of temperature controlled solubility changes in thermonatrite and natron in comparison with other salt phases (solubility data, Schwarz and Stark/Stürmer 1993).
Hygroscopicity[edit]
It is difficult to evaluate the hygroscopicity of sodium carbonates found in situ as the sorption and saturation points are influenced by local conditions, such as presence of other ions and temperature levels which will influence the hydration degree, and therefore can vary considerably.
The table below illustrates the deliquescence humidity of natron in relation to the ambient temperature (see also table Equilibrium humidity in relation to temperature):
| 15°C | 20°C | 25°C | 30°C |
| 96,5% RH | 97,9% RH | 88,2% RH | 83,2% RH |
Crystallization pressure[edit]
The crystallization of thermonatrite from an aqueous solution results in a crystallization pressure of 28.0-33.3 N/mm2, whereas the crystallization pressure for natrite is 7.8-9.2N/mm2 (for comparison, the crystallization pressure data of other masonry decaying salts: 7.2-65.4 N/mm2) [Winkler:1975]Title: Stone: Properties, Durability in Man´s Environment
Author: Winkler, Erhard M. .
.
Hydration behavior[edit]
The compound Na2CO3 and H2O:
Three stable hydrate forms of the Na2CO3 and H2O compound can be found on monuments: the decahydrate, natron, the metastable heptahydrate and, the monohydrate, thermonatrite. The different sodium carbonate hydrates are formed by initiating their precipitation from a supersaturated aqueous solution at different temperatures. Sodium carbonate decahydrate is formed during precipitation at a temperature below 32°C whereas sodium carbonate heptahydrate is formed at a temperature between 32°C and 35.4°C and a solution temperature of above 35.4°C results in the precipitation of sodium carbonate monohydrate, i.e., thermonatrite. These temperatures are called phase transition temperatures. Anhydrous sodium carbonate can be obtained by drying any of the hydrates at approx. 98°C. It will, however, revert to thermonatrite and/or other hydrates at room temperature and normal humidity levels. Since the heptahydrate is formed within a narrow temperature zone of 32°C to 35.4°C, it is relatively unstable under usual climatic conditions (similarly to the anhydrous form of sodium carbonate) and usually occurs as a transitional product during phase changes from the more stable thermonatrite (the monohydrate) to natron (the decahydrate) or vice versa.
Hydration pressure[edit]
Natrite and thermonatrite are considered as damaging to masonry due to their frequent changes in crystal structure and varying water of crystallization content. This occurs as a result of the low hydration/phase transition temperatures of around 35°C illustrated above. The change from thermonatrite to natrite and resultant addition of 9 water molecules into the crystal lattice involves a volume increase of around 260%. The transition from thermonatrite to sodium carbonate heptahydrate (at a temperature of 0-20°C and an RH of approx. 80%) results in a build up of hydration pressure in the region of 28.4 –63.7 N/mm2 [Stark.etal:1996]Title: Bauschädliche Salze
Author: Stark, Jochen; Stürmer, Sylvia .
.
Analytical validation[edit]
Initial identification of thermonatrite and natron can be carried out in situ with the aid of simple solubility tests and pH-measurements: According to Bläuer-Böhm, natron is the only currently known efflorescent salt to have an alkaline pH-value well above 8 but which also displays the ability to deliquesce in its own water of crystallization when heated slightly. Similarly, thermonatrite found in situ also has an alkaline pH-value and is soluble in water. However, thermonatrite is not soluble in water-free ethanol.
Microscopy
[edit]
Laboratory analysis:
The solubility in water and insolubility in ethanol can be confirmed through microscopic examination. Both thermonatrite and natron
have a clear tendency to form needle and/or dendritic crystal shapes during re-crystallization. If gypsum is present in the sample material, a rapid precipitation of calcium carbonate will occur which is visible as a whitish deposit.
Refractive index: nx = 1.420; ny = 1.509; nz = 1.25;
Birefringence: Δ = max. 0.105
Crystal systeme: orthorhombic
Under the polarizing microscope:
The number of crystallization water molecules of the sampled crystals will vary following changes in ambient relative humidity and temperature levels. After only a short time exposed to dry air (with approx. 60% RH) thermonatrite will be found predominantly.
The determination of the refractive index is carried out according to the immersion method. Individual particles soaked in standard immersion oil with a refractive index of nD = 1.518 display a strong change in relief during rotation under transmitted polarized light [Blaeuer-Boehm:1994]Title: Salzuntersuchungen an Baudenkmälern
Author: Bläuer-Böhm, Christine , a.a.O., p. 86. Due to the high birefringence, thermonatrite crystals display bright interference colors. This clearly differentiates it from natrite which has a noticeably smaller maximal birefringence.
, a.a.O., p. 86. Due to the high birefringence, thermonatrite crystals display bright interference colors. This clearly differentiates it from natrite which has a noticeably smaller maximal birefringence.
Thermonatrite belongs to the orthorhombic crystal system. Associated with this is the parallel and/or symmetric extinction when viewed under crossed polars. Larger crystal needles generally appear completely extinguished and the extinction occurs suddenly.
Differentiation of thermonatrite from similar salts:
Thermonatrite is clearly identifiable when the following criteria are known:
- high pH-value
- good solubility in water
- characteristic crystal habit
- all refractive indices under nD=1.53
- high birefringence
- parallel/symmetric extinction
| Salt phase | Differing characteristic in comparison with Thermonatrite |
| Nesquehonite MgCO3 • 3H2O; | poor water solubility / oblique extinction |
| Lansfordite MgCO3 • 5H2O | poor water solubility / oblique extinction / low birefringence |
| Trona Na3H(CO3)2 • 2H2O | usually a noticeable refractive index > 1.53 / oblique extinction |
| Potash K2CO3 | usually a noticeable refractive index > 1.53 / oblique extinction / strongly hygroscopic |
Raman-Spectroscopy[edit]
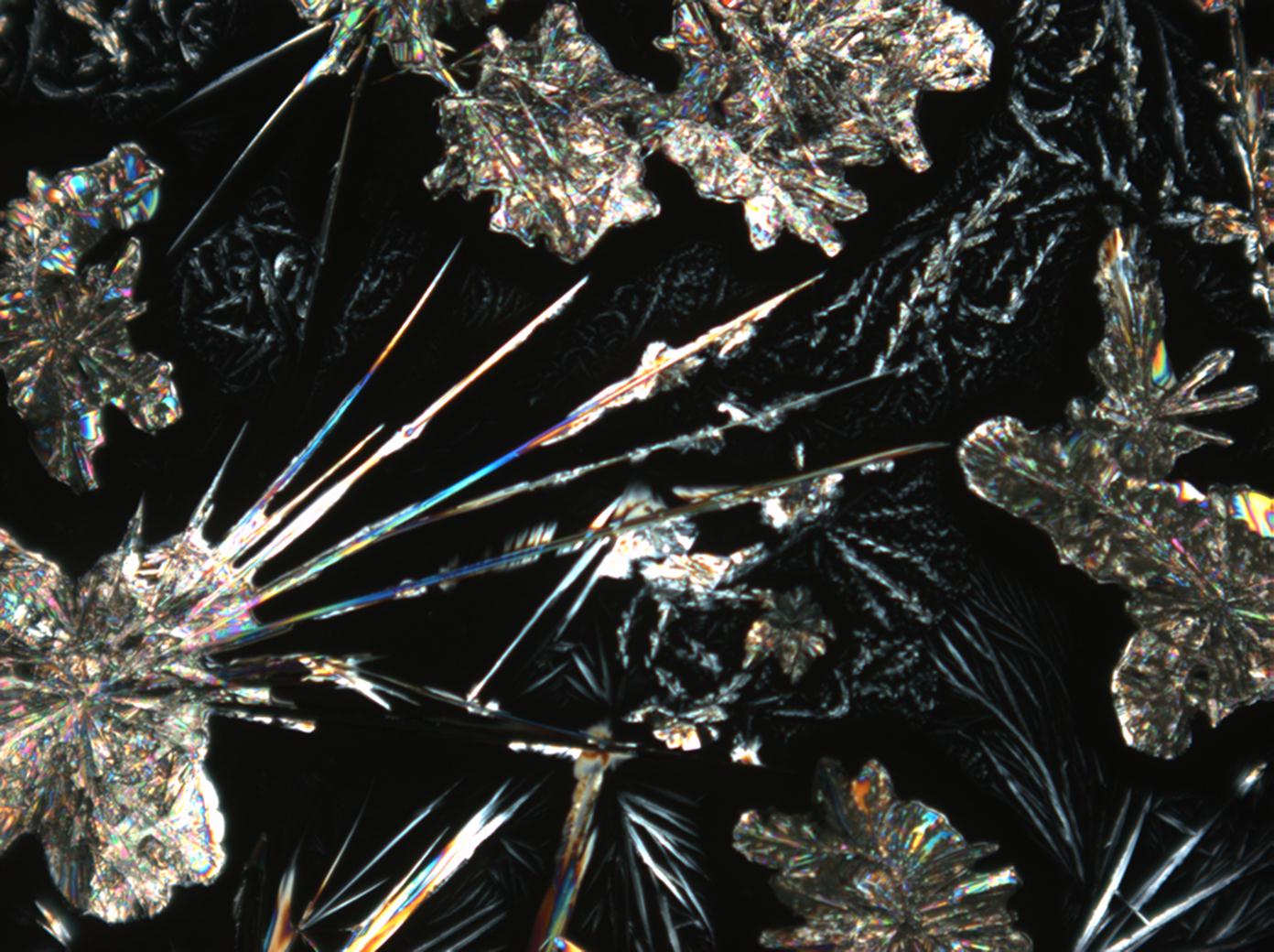
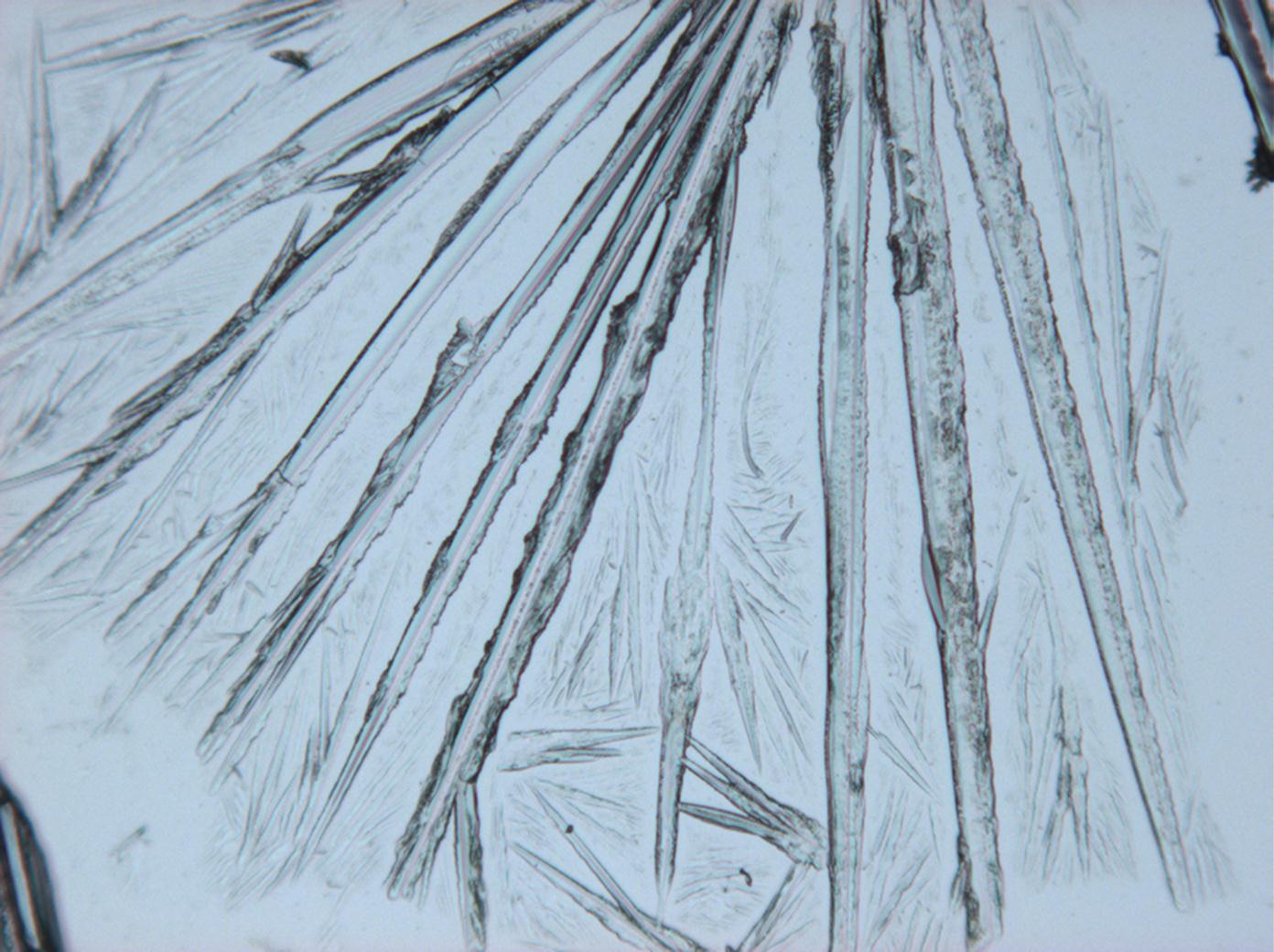
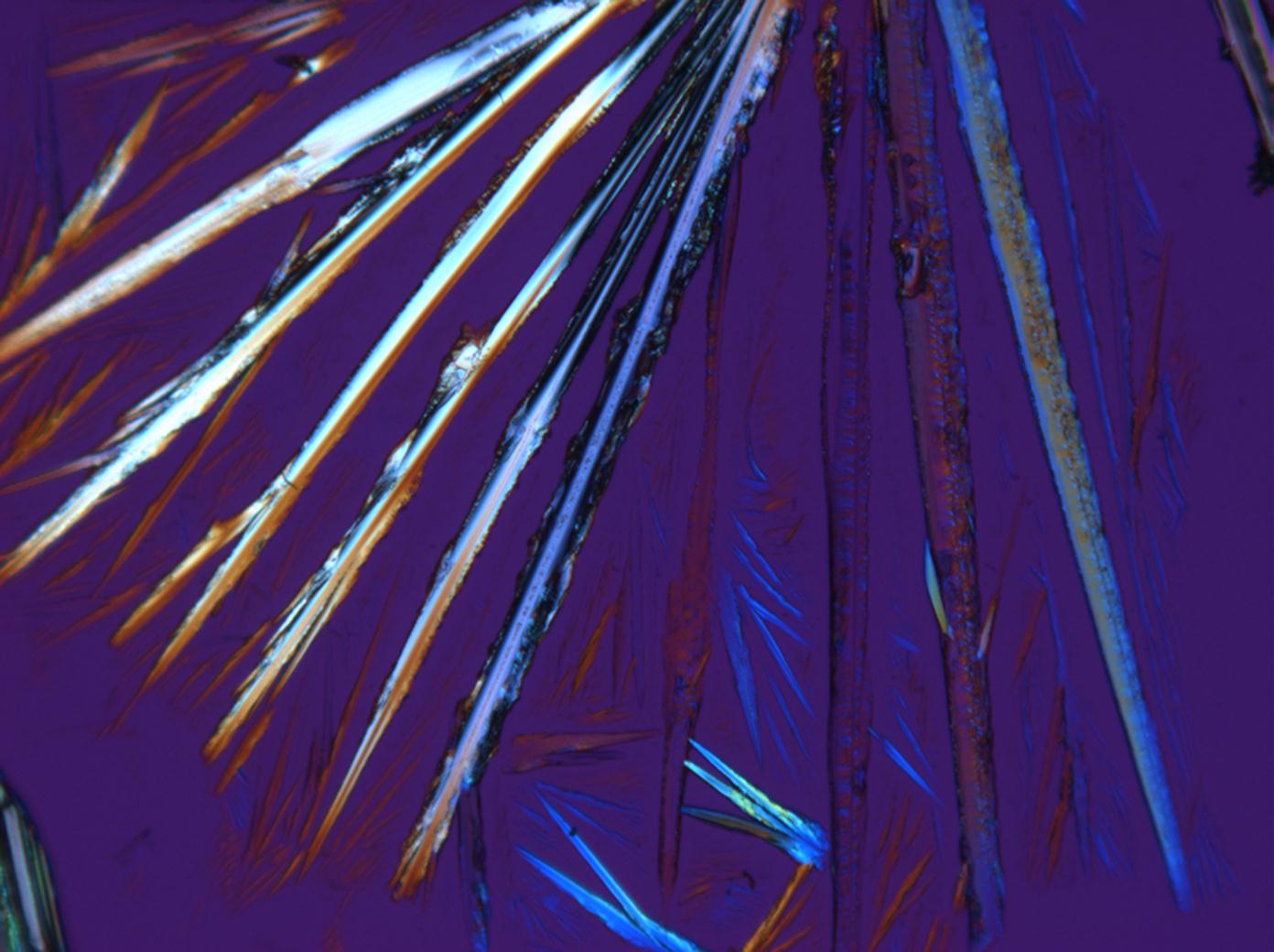
Natrite images[edit]
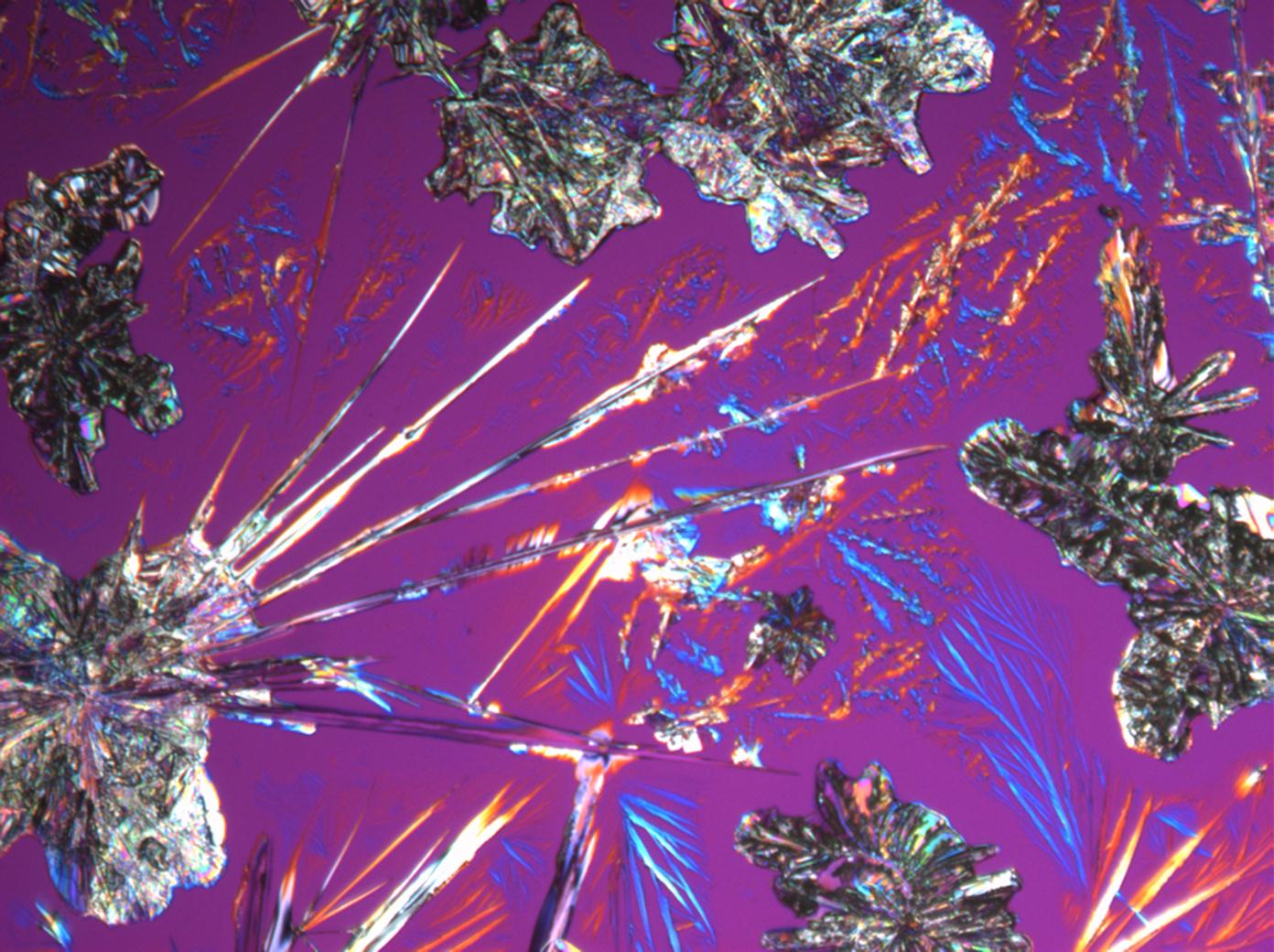
Under the polarizing microscope[edit]
- Na2CO3 from an aqueous solution on a microscope slide
- Na2CO3 from an aqueous solution on a microscope slide
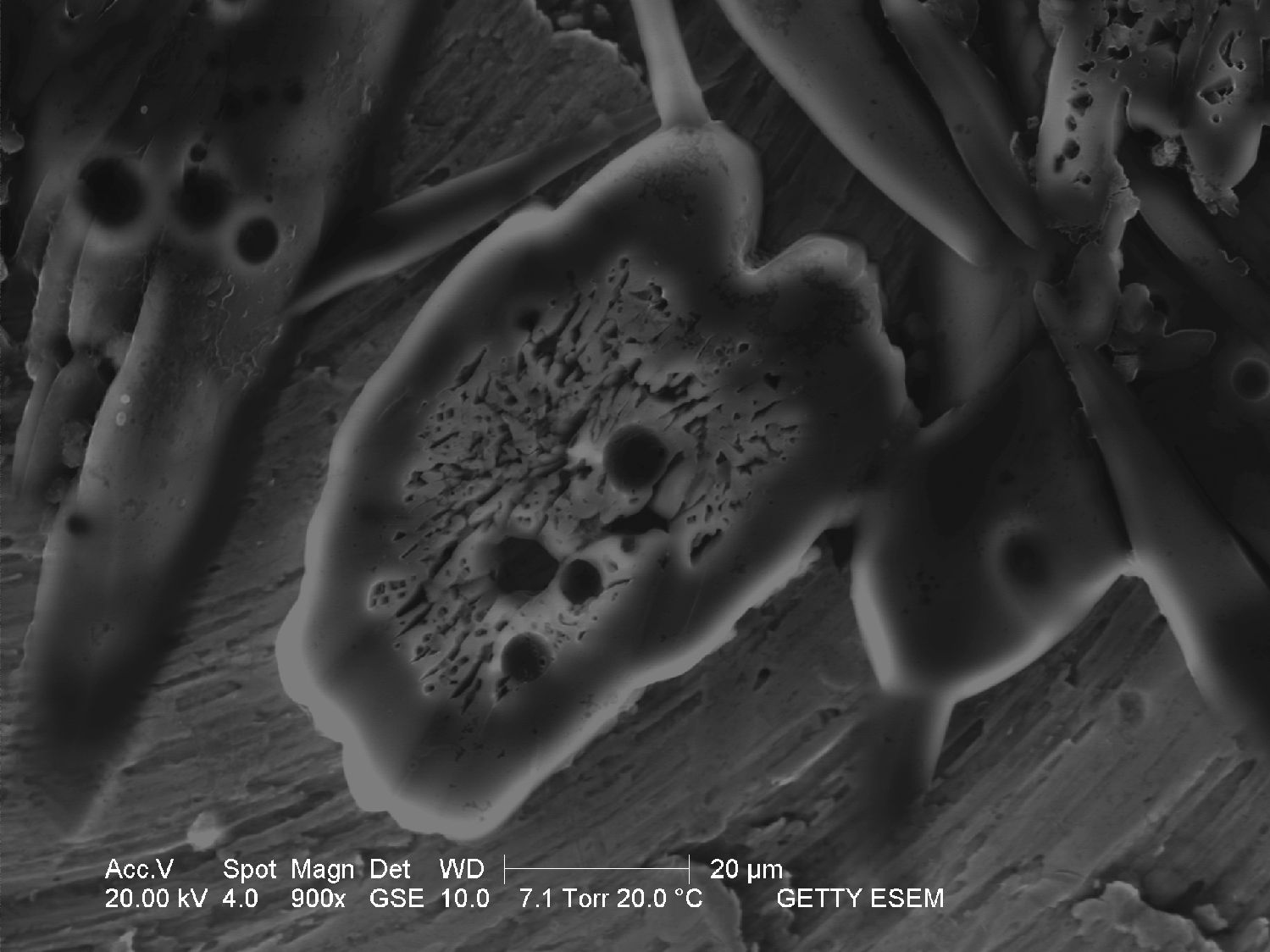
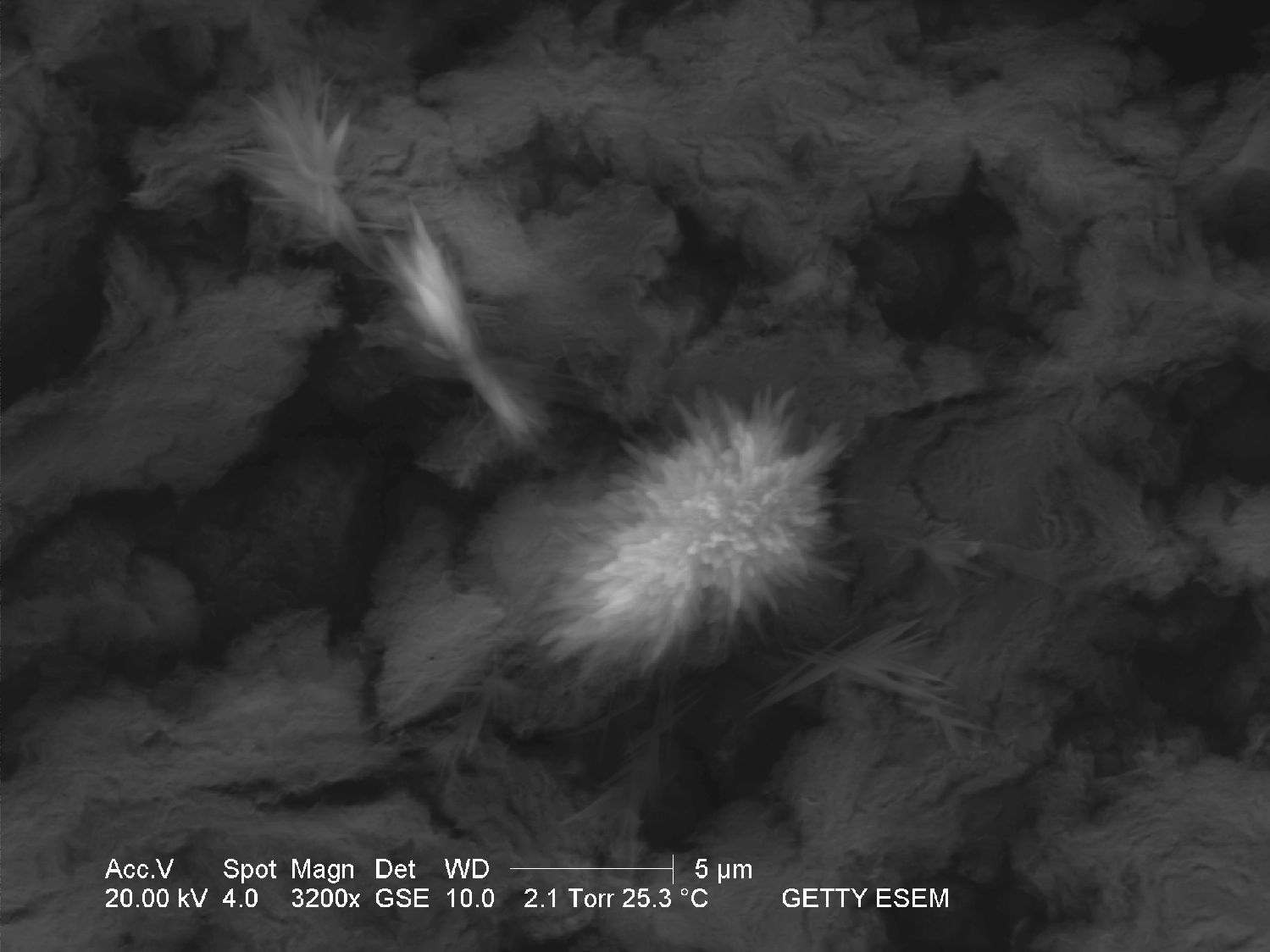
Through the Scanning Electron Microscope[edit]
- Image through SEM
Weblinks[edit]
- ↑ http://webmineral.com/data/Natrite.shtml accessed 09/06/2011
- ↑ http://www.mindat.org/min-2849.html accessed 09/06/2011
- ↑ http://www.chemie.uni-hamburg.de/ac/AKs/Steiger/Raman%_Data%_Base/Na2CO3.htm
Literature[edit]
| [Arnold.etal:1991] | Arnold, Andreas; Zehnder, Konrad (1991): Monitoring Wall Paintings Affected by soluble Salts. In: Cather, Sharon (eds.): The Conservation of Wall Paintings: Proceedings of a symposium organized by the Coutrauld Institut of Art and the Getty Conservation Institute, London, July 13-16, The Getty Conservation Institute, 103-136. |  |
| [Blaeuer-Boehm:1994] | Bläuer-Böhm, Christine (1994): Salzuntersuchungen an Baudenkmälern. In: Zeitschrift für Kunsttechnologie und Konservierung, 8 (1), 86-103 |  |
| [Stark.etal:1996] | Stark, Jochen; Stürmer, Sylvia (1996): Bauschädliche Salze, Bauhaus-Univ. Weimar |  |
| [Winkler:1975] | Winkler, Erhard M. (1975): Stone: Properties, Durability in Man´s Environment, Springer Verlag, Wien |  |
![Raman Spektrum von Natrit - M. Steiger , K. Linnow [3]: Bruker Senterra, 532 nm, 20 mW](https://repository.hawk-hhg.de/images/5/5b/Na2CO3.png)