Antarcticite
| Antarcticite[1][2] | |
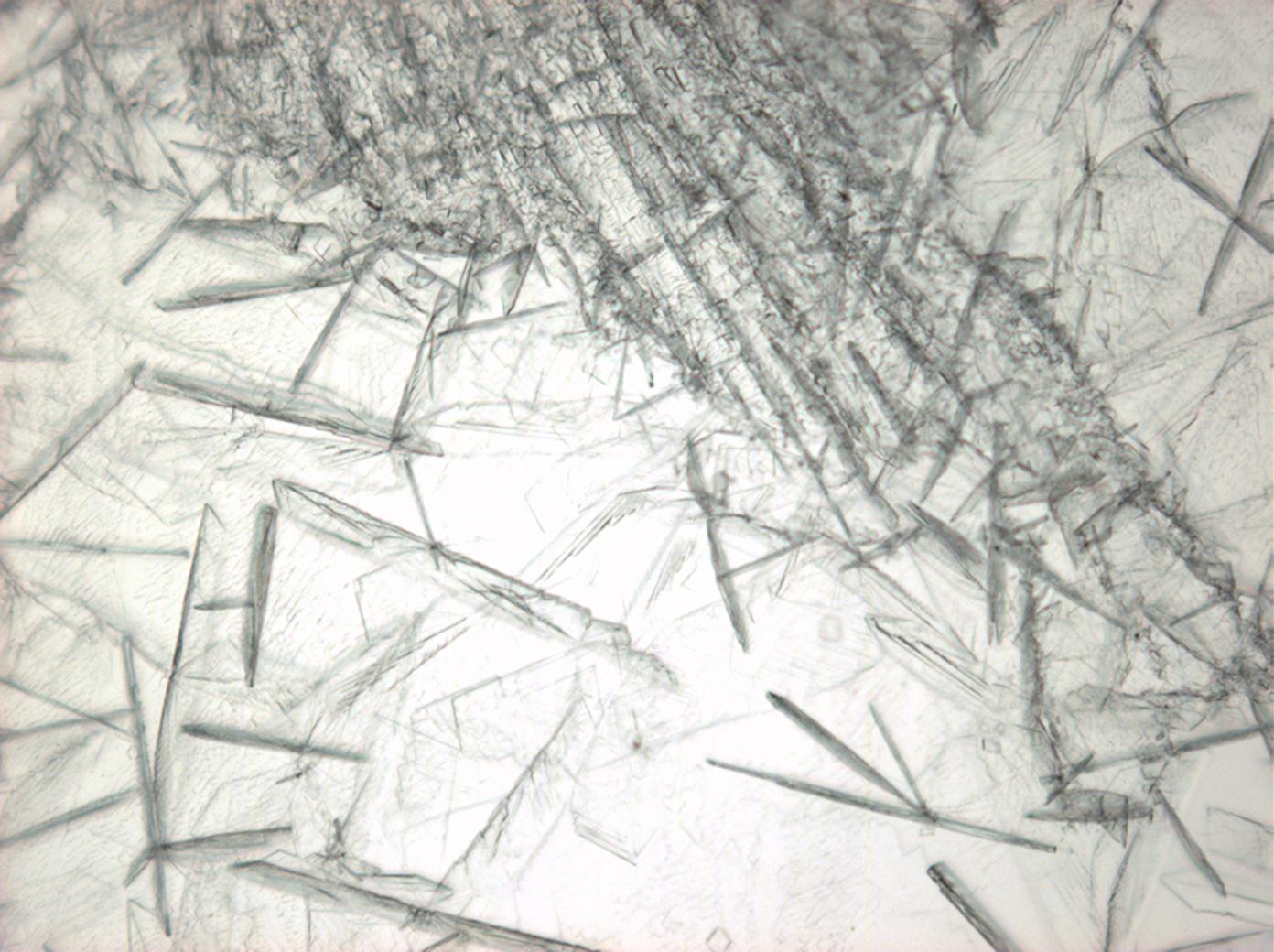
| |
| Mineralogical name | Antarcticite |
| Chemical name | Calcium chloride hexahydrate |
| Trivial name | |
| Chemical formula | CaCl2•6H2O |
| Other forms | |
| Crystal system | trigonal |
| Crystal structure | |
| Deliquescence humidity 20°C | 33.3% [Steiger etal: 2014]Title: Weathering and Deterioration Author: Steiger, Michael; Charola A. Elena; Sterflinger, Katja 
|
| Solubility (g/l) at 20°C | 5360 g/l |
| Density (g/cm³) | 1.68 g/cm³ |
| Molar volume | 128.0 cm3/mol |
| Molar weight | 219.08 g/mol |
| Transparency | transparent |
| Cleavage | perfect |
| Crystal habit | |
| Twinning | |
| Phase transition | |
| Chemical behavior | |
| Comments | hygroscopic and deliquescent |
| Crystal Optics | |
| Refractive Indices | no =1.417-1.494 ne = 1.393-1.550 |
| Birefringence | Δ= 0.024 |
| Optical Orientation | negative |
| Pleochroism | |
| Dispersion | |
| Used Literature | |
| {{{Literature}}} | |
back to Chloride
Hygroscopicity[edit]
| 0°C | 10°C | 20°C | 30°C | 40°C | 50°C |
| 44.3%r.h. | 39.4%r.h. | 33.3%r.h. | 21.6%r.h. (CaCl2•4H2O) | 18.4%r.h. (CaCl2•4H2O) | 16.3%r.h. (CaCl2•2H2O) |
Weblinks[edit]
- ↑ http://webmineral.com/data/Antarcticite.shtml viewed on 29/07/2010
- ↑ http://www.mindat.org/min-251.html viewed on 29/07/2010
Literature[edit]
| [Steiger etal: 2014] | Steiger, Michael; Charola A. Elena; Sterflinger, Katja (2014): Weathering and Deterioration. In:: Stone in Architecture, Siegesmund S. and Snethlage R., 10.1007/978-3-642-45155-3_4. |  |