Calcium chloride: Difference between revisions
| Line 24: | Line 24: | ||
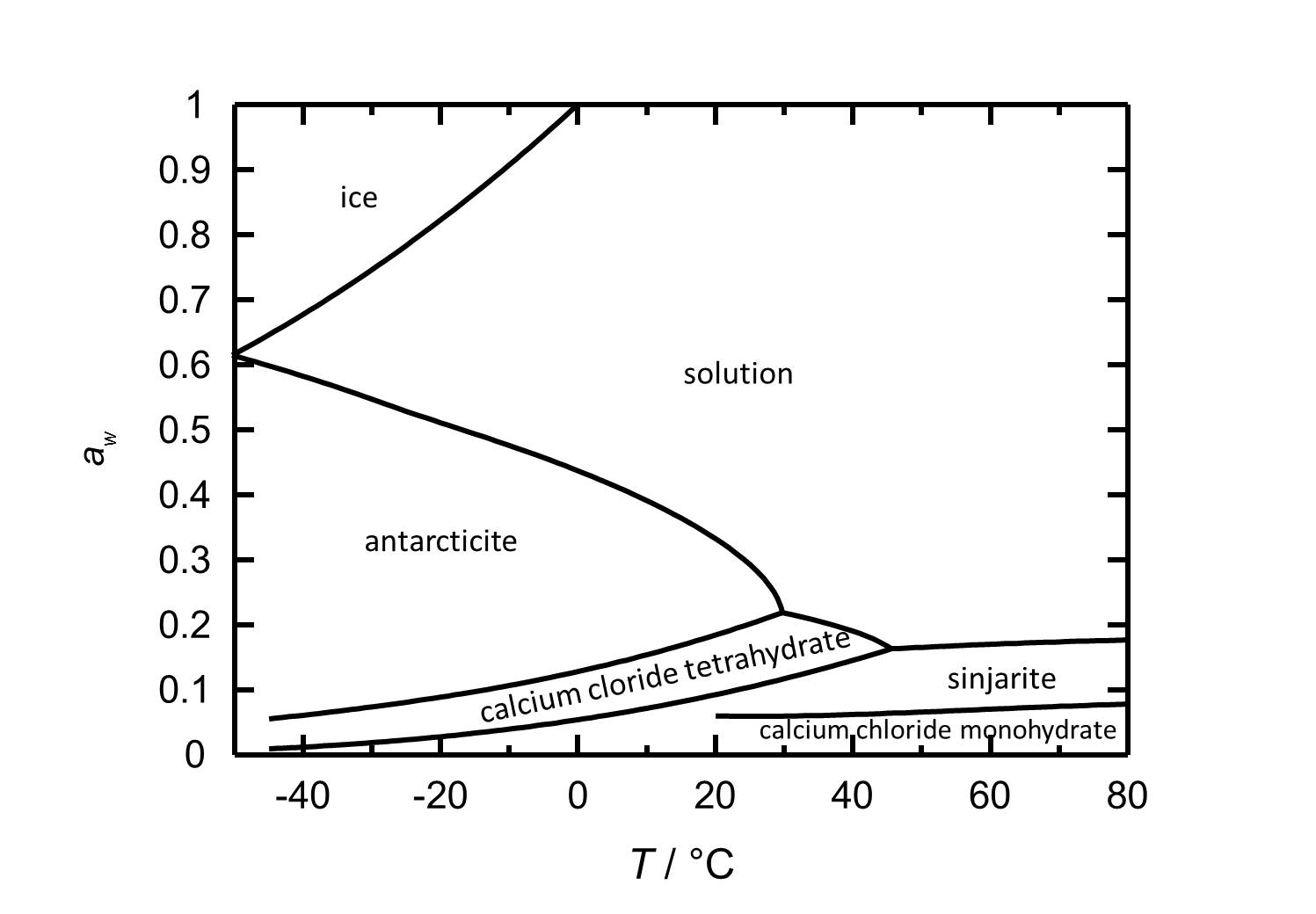
[[file:D CaCl2 e.jpg|thumb|left|800px|Figure 2: Deliquescence behaviour of calcium chloride in the temperature range from -45 to 80 °C. The water activity ''a<sub>w</sub>'' is plotted against the temperature.]] | [[file:D CaCl2 e.jpg|thumb|left|800px|Figure 2: Deliquescence behaviour of calcium chloride in the temperature range from -45 to 80 °C. The water activity ''a<sub>w</sub>'' is plotted against the temperature.]] | ||
<br clear=all> | <br clear=all> | ||
At room temperature the present phase [[Antarcticite]] has got a deliquescence humidity of about 30 %. The deliquescence behavior depends on the temperature as the deliquescence humidity decreases with increasing temperature. Changing the relative humidity at 20 °C, calcium chloride tetrahydrate forms at values below 18 % RH. The dehydration to the Dihydrate ([[sinjarite]]) and the monohydrate occurs at 9 % RH and 6 % RH, respectively. | In the system CaCl<sub>2</sub>-H<sub>2</sub>O hydration/dehydration and crystallization/deliquescence processes can occur by changing relative humidity or temperature. At room temperature the present phase [[Antarcticite]] has got a deliquescence humidity of about 30 %. The deliquescence behavior depends on the temperature as the deliquescence humidity decreases with increasing temperature. Changing the relative humidity at 20 °C, calcium chloride tetrahydrate forms at values below 18 % RH. The dehydration to the Dihydrate ([[sinjarite]]) and the monohydrate occurs at 9 % RH and 6 % RH, respectively. | ||
<br clear="all"> | |||
{|border="2" cellspacing="0" cellpadding="4" width="30%" align="left" class="wikitable" | |||
|+''Table 2: Deliquescence and equilibrium humidities at 20 °C.'' | |||
|- | |||
|bgcolor = "#F0F0F0" align=center| '''Phase transition''' | |||
|bgcolor = "#F0F0F0" align=center| '''Deliquescence or equilibrium humidity at 20°C''' | |||
|- | |||
|bgcolor = "#FFFFEO" align=center| [[Antarcticite]]-solution | |||
|bgcolor = "#FFFFEO" align=center| 33.3 % | |||
|- | |||
|bgcolor = "#FFFFEO" align=center| [[Antarcticite]]-[[Calcium chloride tetrahydrate]] | |||
|bgcolor = "#FFFFEO" align=center| 18.5 % | |||
|- | |||
|bgcolor = "#FFFFEO" align=center| [[Calcium chloride tetrahydrate]]-[[Sinjarite]] | |||
|bgcolor = "#FFFFEO" align=center| 9 % | |||
|- | |||
|bgcolor = "#FFFFEO" align=center| [[Sinjarite]]-[[Calcium chloride monohydrate]] | |||
|bgcolor = "#FFFFEO" align=center| 6 % | |||
|} | |||
<br clear="all"> | |||
==References== | ==References== | ||
Revision as of 12:24, 2 November 2015
Author: Amelie Stahlbuhk
back to Chloride
Abstract[edit]
The different hydrate stages of calcium chloride are presented, as well as their behavior regarding solubility and hygroscopicity.
Hydrate stages[edit]
Sinjarite: CaCl2•2H2O
Calcium chloride tetrahydrate: CaCl2•4H2O
Antarcticite: CaCl2•6H2O
Solubility[edit]
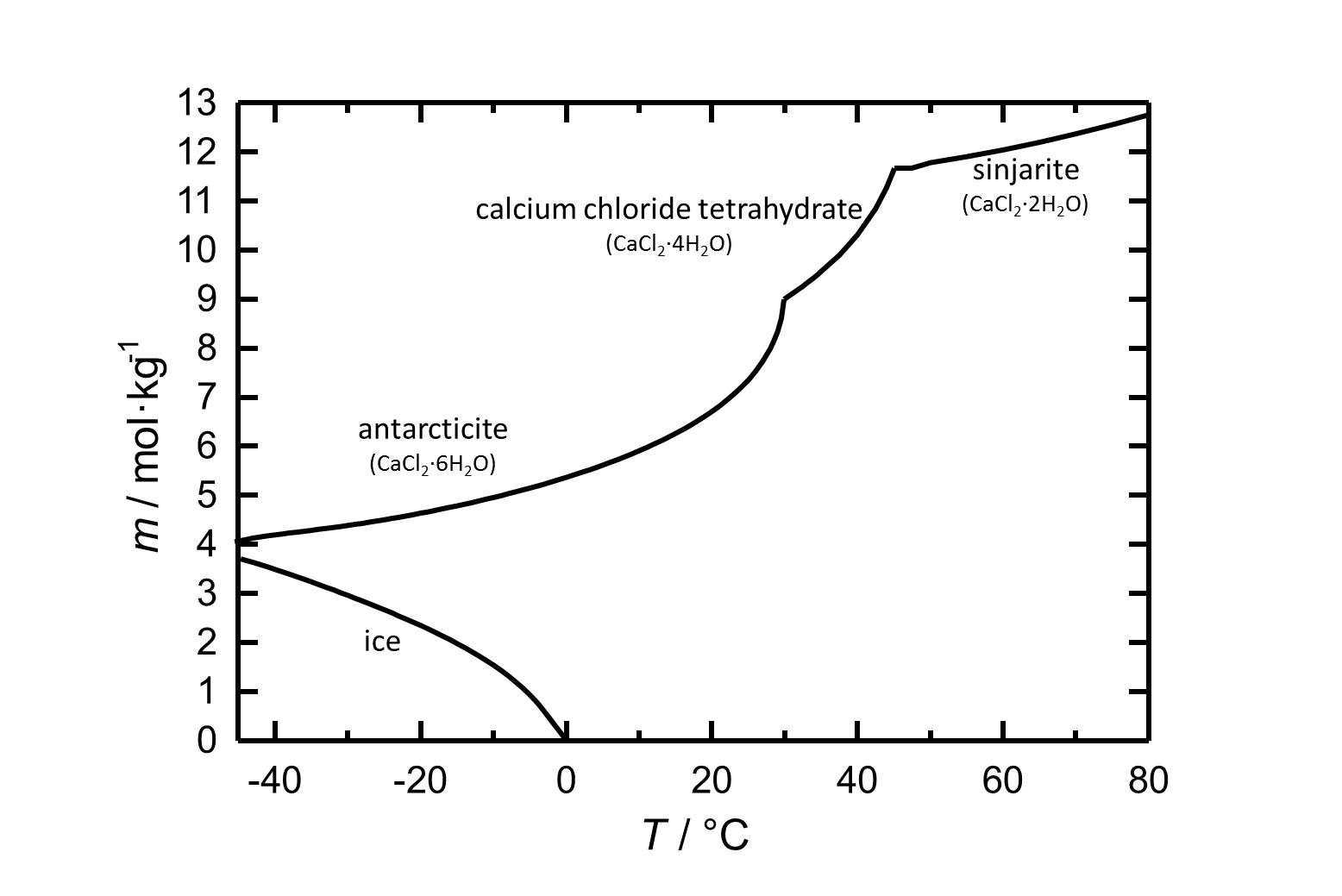
Under standard conditions the hexahydrate of calcium chloride Antarcticite is the stable form. The salt has got a high solubility in water which increases with increasing temperatures. The dehydration steps to the calcium chloride tetrahydrate and to sinjarite take place at temperatures of 30 °C and 45 °C, respectively.
Hygroscopicity[edit]
In the system CaCl2-H2O hydration/dehydration and crystallization/deliquescence processes can occur by changing relative humidity or temperature. At room temperature the present phase Antarcticite has got a deliquescence humidity of about 30 %. The deliquescence behavior depends on the temperature as the deliquescence humidity decreases with increasing temperature. Changing the relative humidity at 20 °C, calcium chloride tetrahydrate forms at values below 18 % RH. The dehydration to the Dihydrate (sinjarite) and the monohydrate occurs at 9 % RH and 6 % RH, respectively.
| Phase transition | Deliquescence or equilibrium humidity at 20°C |
| Antarcticite-solution | 33.3 % |
| Antarcticite-Calcium chloride tetrahydrate | 18.5 % |
| Calcium chloride tetrahydrate-Sinjarite | 9 % |
| Sinjarite-Calcium chloride monohydrate | 6 % |