Natrite: Difference between revisions
Jump to navigation
Jump to search
m (Protected "Natrite" ([edit=sysop] (indefinite) [move=sysop] (indefinite))) |
No edit summary |
||
| Line 11: | Line 11: | ||
|Deliqueszenzhumidity = | |Deliqueszenzhumidity = | ||
|Solubility =210 g/l | |Solubility =210 g/l | ||
|Density =2 | |Density =2.532 g/cm<sup>3</sup> | ||
|MolVolume = | |MolVolume = | ||
|Molweight =105 | |Molweight =105.99 g/mol | ||
|Transparency = | |Transparency = | ||
|Cleavage = | |Cleavage = | ||
|Crystal_Habit = | |Crystal_Habit = | ||
|Twinning = | |Twinning = | ||
|Refractive_Indices =n<sub>x</sub> = 1 | |Refractive_Indices =n<sub>x</sub> = 1.415<br>n<sub>y</sub> = 1.535<br>n<sub>z</sub> = 1.546 | ||
|Birefringence =Δ = 0 | |Birefringence =Δ = 0.131 | ||
|optical_Orientation =biaxial negative | |optical_Orientation =biaxial negative | ||
|Pleochroism = | |Pleochroism = | ||
Revision as of 15:01, 20 December 2011
| Natrite[1][2] | |
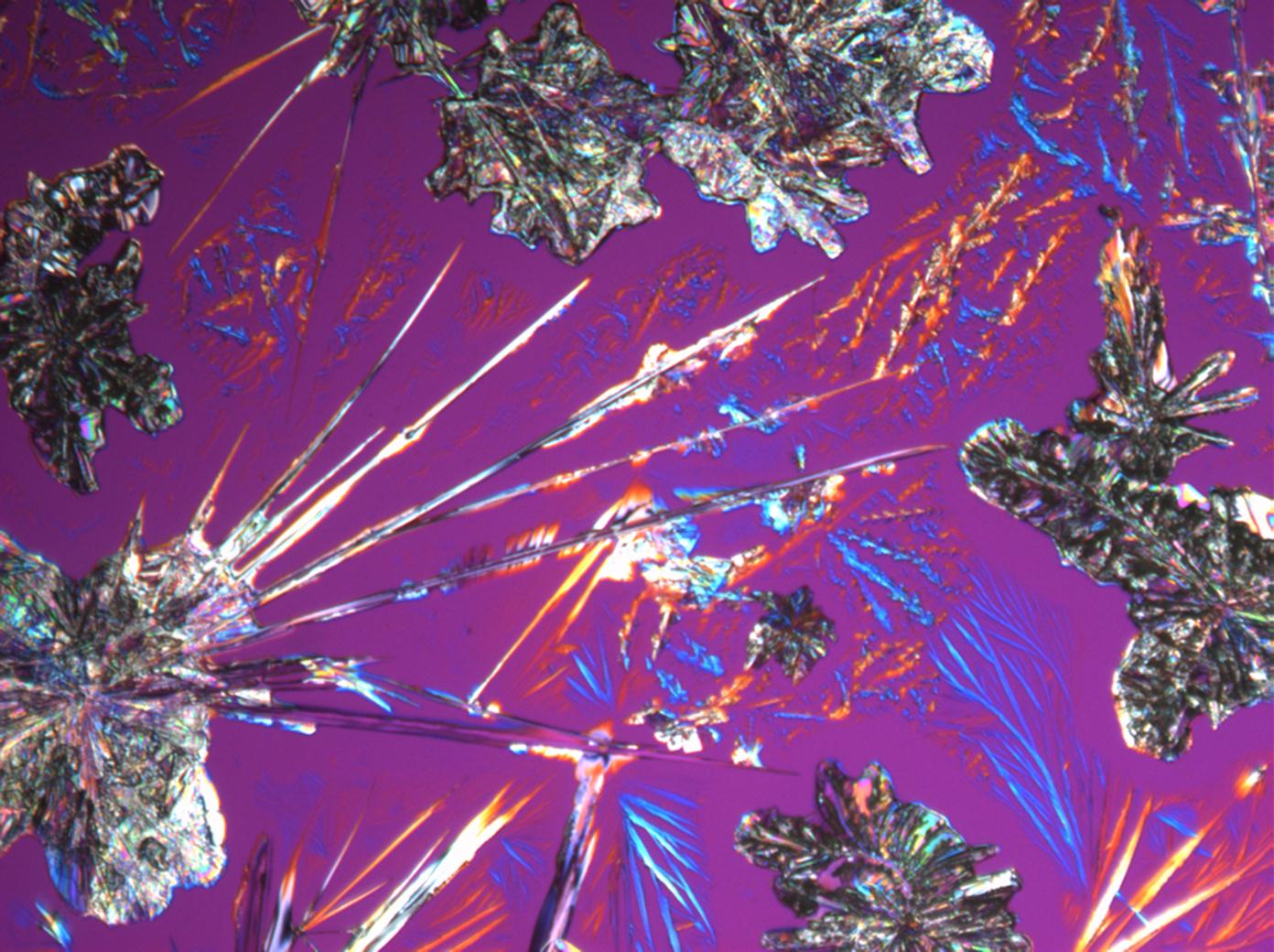
| |
| Mineralogical name | Natrite |
| Chemical name | Sodium carbonate |
| Trivial name | |
| Chemical formula | Na2CO3 |
| Other forms | Sodium carbonate hydrate (Na2CO3•H2O) Sodium carbonate heptahydrate (Na2CO3•7H2O) Sodium carbonate decahydrate (Na2CO3•10H2O) |
| Crystal system | monoclinic |
| Crystal structure | |
| Deliquescence humidity 20°C | |
| Solubility (g/l) at 20°C | 210 g/l |
| Density (g/cm³) | 2.532 g/cm3 |
| Molar volume | |
| Molar weight | 105.99 g/mol |
| Transparency | |
| Cleavage | |
| Crystal habit | |
| Twinning | |
| Phase transition | |
| Chemical behavior | |
| Comments | blooms quickly in dry air and passes into the monohydrate pH about 12 |
| Crystal Optics | |
| Refractive Indices | nx = 1.415 ny = 1.535 nz = 1.546 |
| Birefringence | Δ = 0.131 |
| Optical Orientation | biaxial negative |
| Pleochroism | |
| Dispersion | |
| Used Literature | |
| {{{Literature}}} | |
back to Carbonate
Weblinks[edit]
- ↑ http://webmineral.com/data/Natrite.shtml viewed on 09/06/2011
- ↑ http://www.mindat.org/min-2849.html viewed on 09/06/2011