Bischofite
Jump to navigation
Jump to search
back to Chloride
| Bischofite[1][2] | |
| Mineralogical name | Bischofite |
| Chemical name | Magnesium chloride hexahydrate |
| Trivial name | |
| Chemical formula | MgCl2•6H2O |
| Other forms | |
| Crystal system | monoclinic |
| Crystal structure | |
| Deliquescence humidity 20°C | 33.1% |
| Solubility (g/l) at 20°C | 5.75 mol/kg |
| Density (g/cm³) | 1.57 g/cm3 |
| Molar volume | 129.6 cm3/mol |
| Molar weight | 203.30 g/mol |
| Transparency | transparent to translucent |
| Cleavage | none |
| Crystal habit | |
| Twinning | |
| Phase transition | |
| Chemical behavior | |
| Comments | decomposes at 116-118°C hygroscopic and deliquescent |
| Crystal Optics | |
| Refractive Indices | nx =1.495 ny = 1.509 nz = 1.528 |
| Birefringence | Δ = 0.003 |
| Optical Orientation | positive |
| Pleochroism | |
| Dispersion | 79° |
| Used Literature | |
| [Broul.etal:1981]Title: Solubility in organic two component systems Author: Broul M., Nyvlt J.; Soehnel O.  [Dana:1951]Title: Dana's System of Mineralogy [Dana:1951]Title: Dana's System of MineralogyAuthor: Dana J.D.  [Steiger.etal:2011a]Title: Decomposition reactions of magnesium sulfate hydrates and phase equilibria in the MgSO4-H2O and Na+-Mg2+-Cl--SO42--H2O systems with implications for Mars [Steiger.etal:2011a]Title: Decomposition reactions of magnesium sulfate hydrates and phase equilibria in the MgSO4-H2O and Na+-Mg2+-Cl--SO42--H2O systems with implications for MarsAuthor: Steiger, Michael; Linnow, Kirsten; Ehrhardt, Dorothee; Rohde, Mandy 
| |
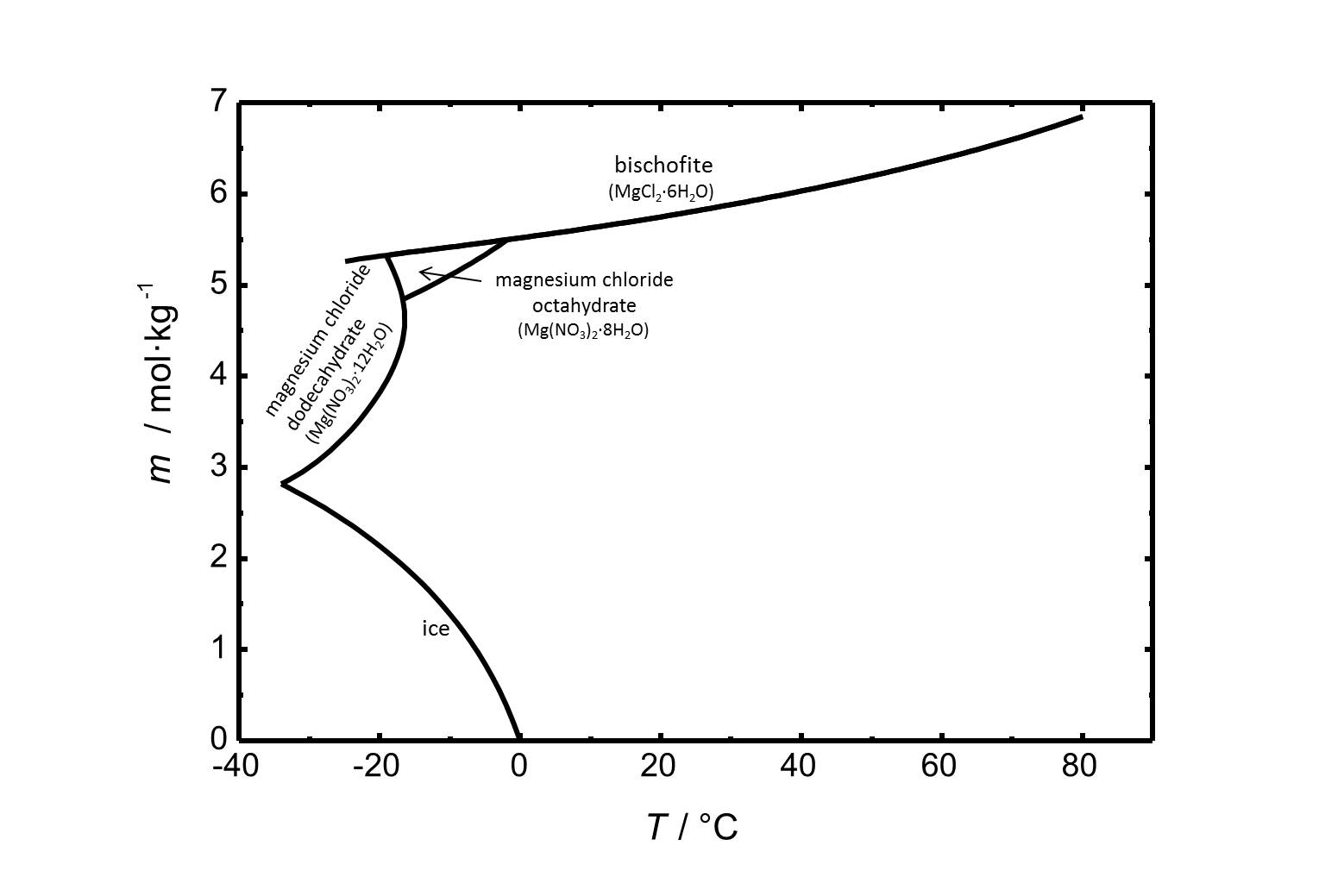
Solubility[edit]
Bischofite has got a high solubility in water (5.75 mol/kg at 20 °C). The solubility of bischofite and other phases of the system MgCl2-H2O is shown in Fig. 1.
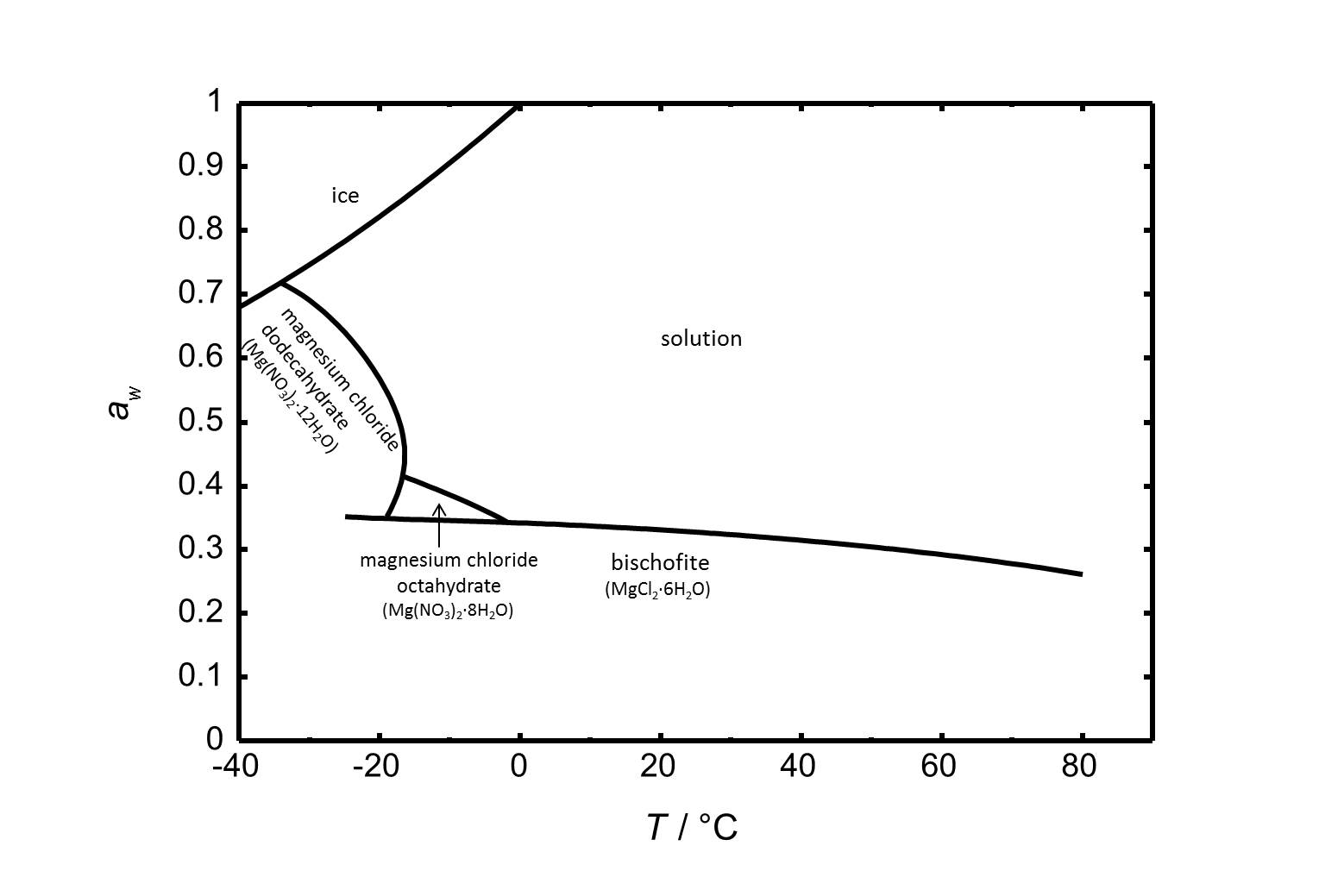
Hygroscopicity[edit]
Figure 2 includes the phase diagram of the system MgCl2-H2O. Next to the hexahydrate bischofite there are two more stable phases within the regarded temperature range. The octa- and the dodecahydrate are relevant at lower temperatures.
| 0°C | 10°C | 20°C | 30°C | 40°C | 50°C |
| 34.1%r.h. | 33.7%r.h. | 33.1%r.h. | 32.4%r.h. | 31.5%r.h. | 30.5%r.h. |
Weblinks[edit]
- ↑ http://webmineral.com/data/Bischofite.shtml viewed on 29/07/2010
- ↑ http://www.mindat.org/min-681.html viewed on 29/07/2010
Literature[edit]
| [Broul.etal:1981] | Elsevier (eds.) Broul M., Nyvlt J.; Soehnel O. (1981): Solubility in organic two component systems, Elsevier |  |
| [Dana:1951] | Dana E.S. (eds.) Dana J.D. (1951): Dana's System of Mineralogy, 7, Wiley & Sons |  |
| [Steiger.etal:2011a] | Steiger, Michael; Linnow, Kirsten; Ehrhardt, Dorothee; Rohde, Mandy (2011): Decomposition reactions of magnesium sulfate hydrates and phase equilibria in the MgSO4-H2O and Na+-Mg2+-Cl--SO42--H2O systems with implications for Mars. In: Geochimica et Cosmochimica Act, 75 (12), 3600-3626, 10.1016/j.gca.2011.03.038, |  |
| [Steiger.etal:2014] | Steiger, Michael; Charola A. Elena; Sterflinger, Katja (2014): Weathering and Deterioration. In: Siegesmund S.; Snethlage R. (eds.): Stone in Architecture, Springer Verlag Berlin Heidelberg, 223-316, 10.1007/978-3-642-45155-3_4. |  |