Sylvite
Jump to navigation
Jump to search
| Sylvite[1][2] | |

| |
| Mineralogical name | Sylvite, Hövelite |
| Chemical name | Potassium chloride |
| Trivial name | |
| Chemical formula | KCl |
| Other forms | |
| Crystal system | cubic |
| Crystal structure | |
| Deliquescence humidity 20°C | 85.0% |
| Solubility (g/l) at 20°C | 4.595 mol/kg |
| Density (g/cm³) | 1.987 g/cm3 |
| Molar volume | 37.52 cm3/mol |
| Molar weight | 74.56 g/mol |
| Transparency | transparent to translucent |
| Cleavage | perfect |
| Crystal habit | |
| Twinning | |
| Phase transition | |
| Chemical behavior | |
| Comments | |
| Crystal Optics | |
| Refractive Indices | n=1.4903 |
| Birefringence | |
| Optical Orientation | isotropic |
| Pleochroism | |
| Dispersion | |
| Used Literature | |
| [Steiger.etal:2014]Title: Weathering and Deterioration Author: Steiger, Michael; Charola A. Elena; Sterflinger, Katja  [Robie.etal:1978]Title: Thermodynamic properties of minerals and related substances at 298.15 K and 1 bar pressure and higher temperatures [Robie.etal:1978]Title: Thermodynamic properties of minerals and related substances at 298.15 K and 1 bar pressure and higher temperaturesAuthor: Robie R.A., Hemingway B.S.; Fisher J.A.  [Dana:1951]Title: Dana's System of Mineralogy [Dana:1951]Title: Dana's System of MineralogyAuthor: Dana J.D. 
| |
back to Chloride
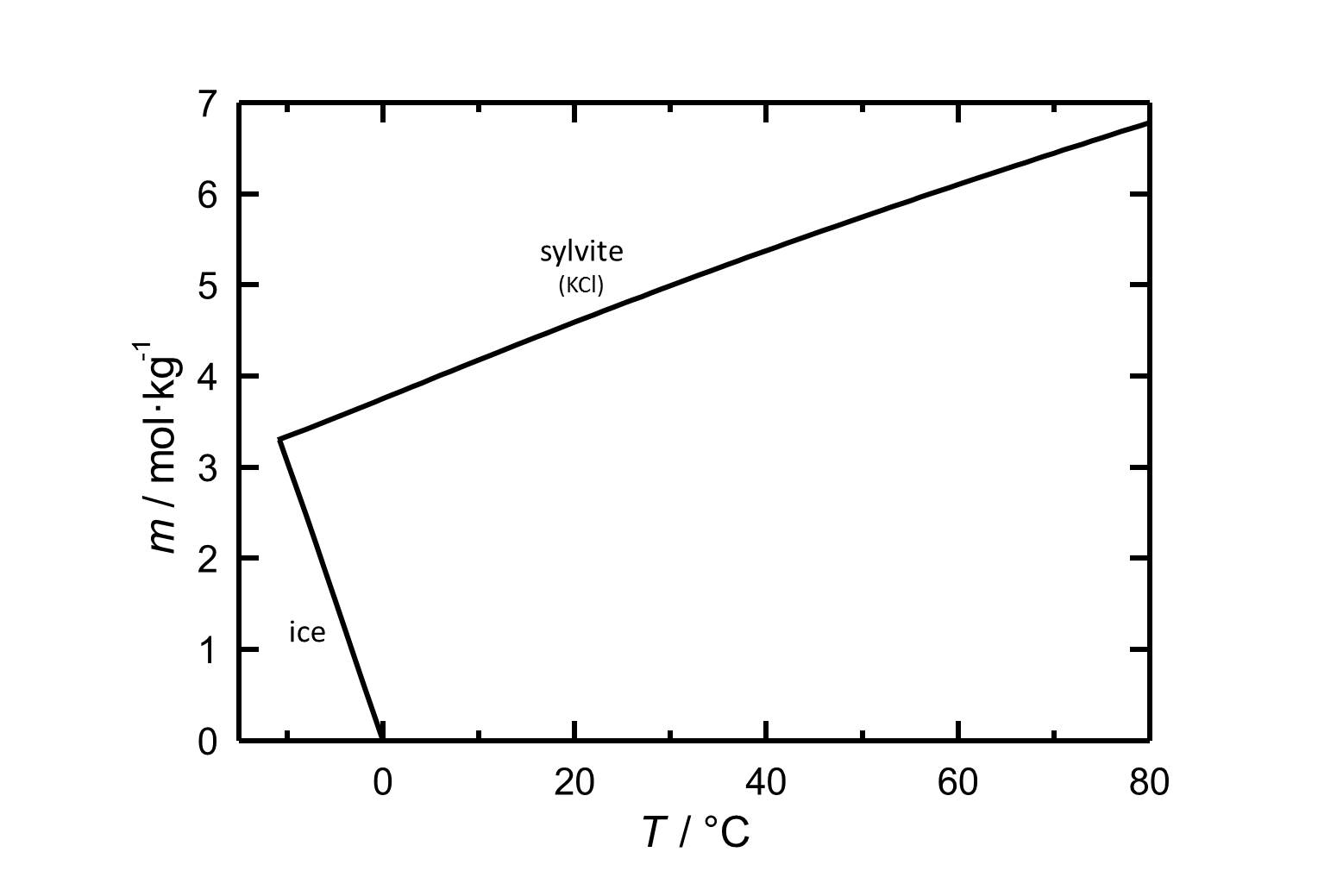
Solubility[edit]

Figure 1: Solubility of potassium chloride in water. The molality m [n(KCl)•kg(H2O)-1] is plotted versus the temperature. According to [Steiger.etal:2008c]Title: An improved model incorporating Pitzer’s equations for calculation of thermodynamic properties of pore solutions implemented into an efficient program code
Author: Steiger, Michael; Kiekbusch, Jana; Nicolai, Andreas

Author: Steiger, Michael; Kiekbusch, Jana; Nicolai, Andreas

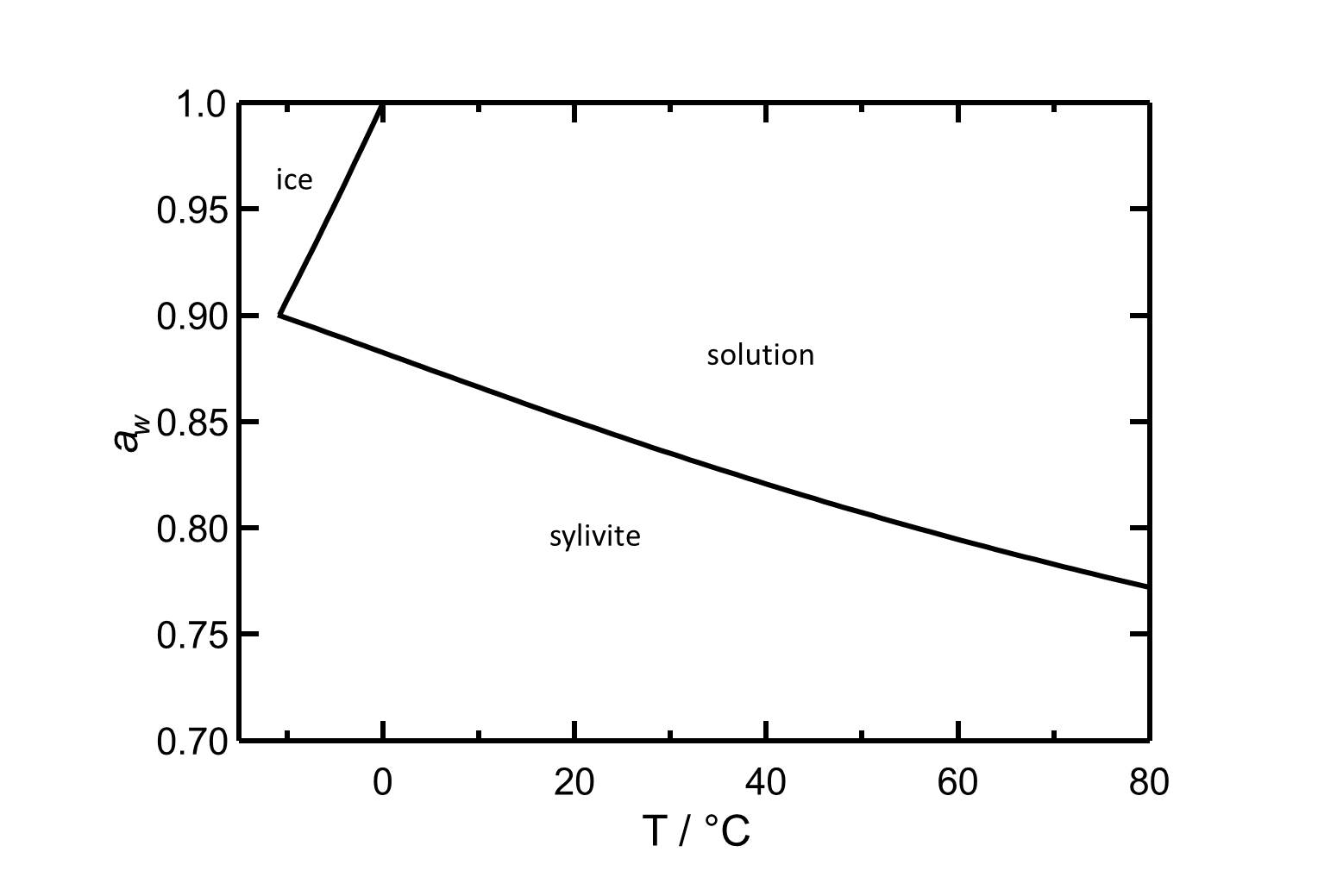
Hygroscopicity[edit]

Figure 2: Deliquescence behaviour of potassium chloride. The water activity aw is plotted versus the temperature. According to [Steiger.etal:2008c]Title: An improved model incorporating Pitzer’s equations for calculation of thermodynamic properties of pore solutions implemented into an efficient program code
Author: Steiger, Michael; Kiekbusch, Jana; Nicolai, Andreas

Author: Steiger, Michael; Kiekbusch, Jana; Nicolai, Andreas

| 0°C | 10°C | 20°C | 30°C | 40°C | 50°C |
| 88.3%r.h. | 86.7%r.h. | 85.0%r.h. | 83.5%r.h. | 82.1%r.h. | 80.7%r.h. |
Weblinks[edit]
- ↑ http://webmineral.com/data/Sylvite.shtml viewed on 29/07/2010
- ↑ http://www.mindat.org/min-3850.html viewed on 29/07/2010
Literature[edit]
| [Dana:1951] | Dana E.S. (eds.) Dana J.D. (1951): Dana's System of Mineralogy, 7, Wiley & Sons |  |
| [Robie.etal:1978] | Robie R.A., Hemingway B.S.; Fisher J.A. (1978): Thermodynamic properties of minerals and related substances at 298.15 K and 1 bar pressure and higher temperatures. In: U.S. Geol. Surv. Bull, 1452 () |  |
| [Steiger.etal:2008c] | Steiger, Michael; Kiekbusch, Jana; Nicolai, Andreas (2008): An improved model incorporating Pitzer’s equations for calculation of thermodynamic properties of pore solutions implemented into an efficient program code. In: Construction and Building Materials, 22 (8), 1841-1850, 10.1016/j.conbuildmat.2007.04.020 |  |
| [Steiger.etal:2014] | Steiger, Michael; Charola A. Elena; Sterflinger, Katja (2014): Weathering and Deterioration. In: Siegesmund S.; Snethlage R. (eds.): Stone in Architecture, Springer Verlag Berlin Heidelberg, 223-316, 10.1007/978-3-642-45155-3_4. |  |