Sodium sulfate
Authors: Hans-Jürgen Schwarz , Michael Steiger, Tim Müller, Amelie Stahlbuhk
back to Sulfate
Abstract[edit]
In this article the different phases of sodium sulfate and their properties will be presented.
Phases and hydrate phases[edit]
There are four different phases of sodium sulfate whereof only two are stable. The others are metastable but were also detected.
Thenardite Na2SO4
Sodium sulfate phase III Na2SO4 metastable
Sodium sulfate heptahydrate Na2SO4•7H2O metastable
Mirabilite Na2SO4•10H2O
Solubility[edit]
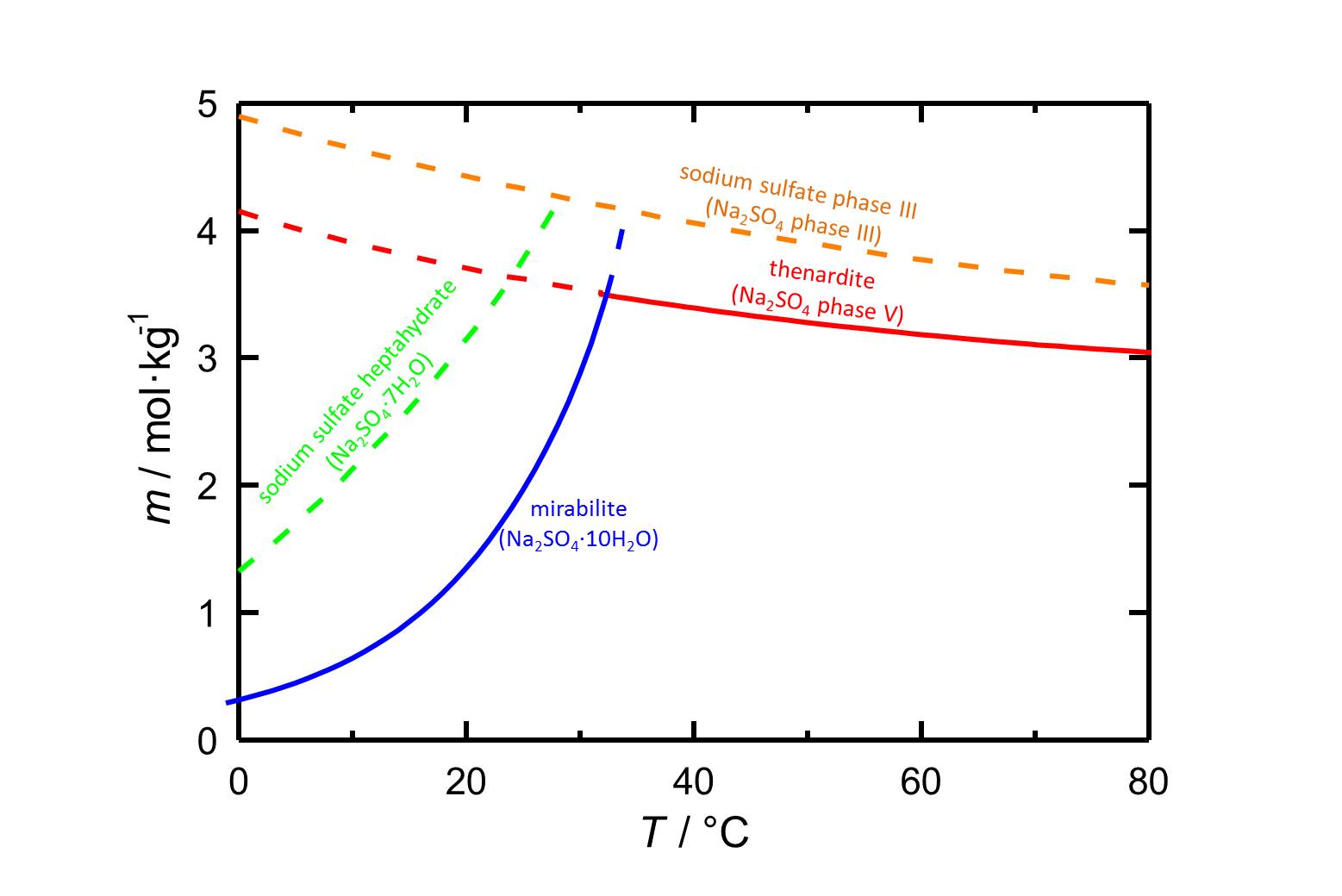
Author: Steiger, Michael; Asmussen, Sönke
 .
.
The phases of sodium sulfate are easily soluble in water (figure 1), so they have a high mobility in porous materials as well. The solubility of the phases depends on the temperature.
The decahydrate mirabilite is stable below 32.4 °C. Above this temperature the anhydrous thenardite is the stable crystalline phase, which in turn is metastable below this transition temperature. In the case of a temperature dropin a solution that is saturated with respect to thenardite, high supersaturations with respect to mirabilite and with that its precipitation are possible, which involves a certain damage potential.
| phase | solubility [mol/kg] at 20°C |
| thenardite | 3.706 |
| sodium sulfate phase III | 4.428 |
| sodium sulfate heptahydrate | 3.143 |
| mirabilite | 1.353 |