Kieserite: Difference between revisions
No edit summary |
No edit summary |
||
| (5 intermediate revisions by 2 users not shown) | |||
| Line 4: | Line 4: | ||
{{Infobox_Salt | {{Infobox_Salt | ||
|Footnote = <ref>http://webmineral.com/data/Kieserite.shtml viewed on 29/07/2010</ref><ref>http://www.mindat.org/min-2204.html viewed on 29/07/2010 | |Footnote = <ref>http://webmineral.com/data/Kieserite.shtml viewed on 29/07/2010</ref><ref>http://www.mindat.org/min-2204.html viewed on 29/07/2010</ref> | ||
|photo = [[File:HJS MgSO4 092503-3.jpg|300px]] | |photo = [[File:HJS MgSO4 092503-3.jpg|300px]] | ||
|mineralogical_Name = Kieserite | |mineralogical_Name = Kieserite | ||
| Line 10: | Line 10: | ||
|Trivial_Name = | |Trivial_Name = | ||
|chemical_Formula = MgSO<sub>4</sub>•H<sub>2</sub>O | |chemical_Formula = MgSO<sub>4</sub>•H<sub>2</sub>O | ||
|Hydratforms = [[Sanderite]] (MgSO<sub>4</sub>•2H<sub>2</sub>O)<br> [[Starkeyite]] (MgSO<sub>4</sub>•4H<sub>2</sub>O)<br> [[Pentahydrite]] (MgSO<sub>4</sub>•5H<sub>2</sub>O)<br> [[Hexahydrite]] (MgSO<sub>4</sub>•6H<sub>2</sub>O)<br> [[Epsomite]] (MgSO<sub>4</sub>•7H<sub>2</sub>O)<br> [[ | |Hydratforms = [[Sanderite]] (MgSO<sub>4</sub>•2H<sub>2</sub>O)<br> [[Starkeyite]] (MgSO<sub>4</sub>•4H<sub>2</sub>O)<br> [[Pentahydrite]] (MgSO<sub>4</sub>•5H<sub>2</sub>O)<br> [[Hexahydrite]] (MgSO<sub>4</sub>•6H<sub>2</sub>O)<br> [[Epsomite]] (MgSO<sub>4</sub>•7H<sub>2</sub>O)<br> [[Meridianiite]] (MgSO<sub>4</sub>•11 H<sub>2</sub>O)<br> [[Magnesium 12-Hydrate]] | ||
|Crystal_System = monoclinic | |Crystal_System = monoclinic | ||
|Crystal_Structure = monoclinic prismatic | |Crystal_Structure = monoclinic prismatic | ||
| Line 39: | Line 39: | ||
== Occurrence of kieserite == | == Occurrence of kieserite == | ||
Kieserite rarely forms individual crystals. The magnesium sulfate hydrate occurs in natural salt deposits together with [[ | Kieserite rarely forms individual crystals. The magnesium sulfate hydrate occurs in natural salt deposits together with [[sylvite]] and [[halite]]. These can be found in the alps as well as Northern Germany, Italy ( Mt. Vesuvius), Arizona (Bisbee), Nevada and Washington (Mt. Kruger). More commonly [[epsomite]] occurs as efflorescence on magnesian stone. These stones generally also contain significant amounts of carbonate ([[calcite]] or [[dolomite]]). Kieserite is precipitated during the evaporation of liquids and as secondary mineral during the oxidation of iron sulfides. It also forms on natural stone surfaces where it can cause substantial damages. | ||
== Information on the origins and formation of kieserite on monuments <br> == | == Information on the origins and formation of kieserite on monuments <br> == | ||
| Line 45: | Line 45: | ||
[[Epsomite|Magnesium sulfate]] forms on surfaces which offer a source of magnesium ions. These can be found in different materials: | [[Epsomite|Magnesium sulfate]] forms on surfaces which offer a source of magnesium ions. These can be found in different materials: | ||
*Lime with dolomitic content: | *Lime with dolomitic content: | ||
[[Dolomite]] is a double salt which can contain both calcium and magnesium. Carbonate forms the negatively charged ion. Dolomitic lime is used to manufacture mortar. Burning dolomitic limestone and slaking the resultant lime results in the formation of pure salts (CaCO<sub>3</sub> and MgCO<sub>3</sub>). The solubility of [[magnesite]] (1,76 g/l) is noticeably higher than that of the pure [[calcite]] (0,014 g/l) or of [[dolomite]] (0,078 g/l). Dampness in the masonry can cause magnesium bonds to dissolve and salts to re-form on drying. This can lead to the formation of [[sulfates]], especially if the use of gypsum-rich products on the same object offer a ready source of sulfate ions. | [[Dolomite]] is a double salt which can contain both calcium and magnesium. Carbonate forms the negatively charged ion. Dolomitic lime is used to manufacture mortar. Burning dolomitic limestone and slaking the resultant lime results in the formation of pure salts (CaCO<sub>3</sub> and MgCO<sub>3</sub>). The solubility of [[magnesite]] (1,76 g/l) is noticeably higher than that of the pure [[calcite]] (0,014 g/l) or of [[dolomite]] (0,078 g/l). Dampness in the masonry can cause magnesium bonds to dissolve and salts to re-form on drying. This can lead to the formation of [[Sulfate|sulfates]], especially if the use of gypsum-rich products on the same object offer a ready source of sulfate ions. | ||
CaMg[CO<sub>3</sub>]<sub>2</sub> -> CaCO<sub>3</sub> + MgCO<sub>3</sub> | CaMg[CO<sub>3</sub>]<sub>2</sub> -> CaCO<sub>3</sub> + MgCO<sub>3</sub> | ||
| Line 61: | Line 61: | ||
== Solubility behavior == | == Solubility behavior == | ||
The water solubility of kieserite is | [[File:S MgSO4.jpg|thumb|800px|left|'''Figure 1:''' Solubilities in the system MgSO<sub>4</sub>-H<sub>2</sub>O and its temperature dependence. The molality ''m'' [n(MgSO<sub>4</sub>•xH<sub>2</sub>O)•kg(H<sub>2</sub>O)<sup>-1</sup>] is plotted versus the temperature. Equilibria of the different hydrated forms can be distiguished by the different colours of the curves. Dotted lines represent the metastable equilibria. In addition to the solubilities, freezing and boiling points are given. According to <bib id="Steiger.etal:2011a"/>.]] | ||
<br clear="all"> | |||
The water solubility of kieserite is 4.108 mol/kg at a temperature of 100 °C <bib id="Steiger.etal:2011a"/>. It subsequently belongs, like all discussed forms of magnesium sulphate with a solubility of clearly above 100 g/l (at 20 °C) to the group of very water soluble salts. This entails a high risk of salt mobility and frequent re-deposition of salts within the material matrix. Due to the influence of temperature on solubility, a rapid drop in temperature can result in the precipitation of salts <bib id="Mainusch:2001"/> | |||
== Hygroscopicity == | == Hygroscopicity == | ||
The low hygroscopicity of the pure kieserite salt (illustrated by its 42% equilibrium RH) cannot be examined on its own. | The low hygroscopicity of the pure kieserite salt (illustrated by its 42% equilibrium RH) cannot be examined on its own. | ||
[[ | [[File:D MgSO4 e.jpg|thumb|800px|left|'''Figure 2:''' Deliquescence behaviour in the system MgSO<sub>4</sub>-H<sub>2</sub>O. The water activity ''a<sub>w</sub>'' is plotted versus the temperature. Different colours label equilibrium and deliquescence humidities of the different phases of the system. Dotted lines represent metastable equilibria. According to <bib id="Steiger.etal:2011a"/>.]] | ||
<br clear="all"> | |||
The sorption point of mixed systems, i.e. in the presence of other ions, is lower. The possibility of hygroscopic moisture absorption and associated problems is still present, in spite of the high deliquescence point <bib id="Mainusch:2001"/> | The sorption point of mixed systems, i.e. in the presence of other ions, is lower. The possibility of hygroscopic moisture absorption and associated problems is still present, in spite of the high deliquescence point <bib id="Mainusch:2001"/> | ||
Latest revision as of 11:01, 3 May 2023
Authors: Hans-Jürgen Schwarz, Tim Müller
English version by Christa Gerdwilker
back to Sulfate
| Kieserite[1][2] | |
| |
| Mineralogical name | Kieserite |
| Chemical name | Magnesiumsulfate Monohydrate |
| Trivial name | |
| Chemical formula | MgSO4•H2O |
| Other forms | Sanderite (MgSO4•2H2O) Starkeyite (MgSO4•4H2O) Pentahydrite (MgSO4•5H2O) Hexahydrite (MgSO4•6H2O) Epsomite (MgSO4•7H2O) Meridianiite (MgSO4•11 H2O) Magnesium 12-Hydrate |
| Crystal system | monoclinic |
| Crystal structure | monoclinic prismatic |
| Deliquescence humidity 20°C | |
| Solubility (g/l) at 20°C | 5.600 mol/kg |
| Density (g/cm³) | 2.57 g/cm3 |
| Molar volume | 53.85 cm3/mol |
| Molar weight | 138.4 g/mol |
| Transparency | translucent |
| Cleavage | perfect |
| Crystal habit | |
| Twinning | |
| Phase transition | |
| Chemical behavior | |
| Comments | can be produced from an aqueous solution over 67°C |
| Crystal Optics | |
| Refractive Indices | nx = 1.520 ny = 1.533 nz = 1.584 |
| Birefringence | Δ = 0.064 |
| Optical Orientation | biaxial positive |
| Pleochroism | |
| Dispersion | 55° |
| Used Literature | |
| [Broul.etal:1981]Title: Solubility in organic two component systems Author: Broul M., Nyvlt J.; Soehnel O.  [Dana:1951]Title: Dana's System of Mineralogy [Dana:1951]Title: Dana's System of MineralogyAuthor: Dana J.D.  [Friedrich.etal:1961]Title: Identität von Wathlingenit mit Kieserit [Friedrich.etal:1961]Title: Identität von Wathlingenit mit KieseritAuthor: Friedrich K., Kuhn R., Strunz H. 
| |
Abstract[edit]
Overview of the magnesium sulfate ‘kieserite’.
Occurrence of kieserite[edit]
Kieserite rarely forms individual crystals. The magnesium sulfate hydrate occurs in natural salt deposits together with sylvite and halite. These can be found in the alps as well as Northern Germany, Italy ( Mt. Vesuvius), Arizona (Bisbee), Nevada and Washington (Mt. Kruger). More commonly epsomite occurs as efflorescence on magnesian stone. These stones generally also contain significant amounts of carbonate (calcite or dolomite). Kieserite is precipitated during the evaporation of liquids and as secondary mineral during the oxidation of iron sulfides. It also forms on natural stone surfaces where it can cause substantial damages.
Information on the origins and formation of kieserite on monuments
[edit]
Magnesium sulfate forms on surfaces which offer a source of magnesium ions. These can be found in different materials:
- Lime with dolomitic content:
Dolomite is a double salt which can contain both calcium and magnesium. Carbonate forms the negatively charged ion. Dolomitic lime is used to manufacture mortar. Burning dolomitic limestone and slaking the resultant lime results in the formation of pure salts (CaCO3 and MgCO3). The solubility of magnesite (1,76 g/l) is noticeably higher than that of the pure calcite (0,014 g/l) or of dolomite (0,078 g/l). Dampness in the masonry can cause magnesium bonds to dissolve and salts to re-form on drying. This can lead to the formation of sulfates, especially if the use of gypsum-rich products on the same object offer a ready source of sulfate ions.
CaMg[CO3]2 -> CaCO3 + MgCO3
MgCO3 + n H2O -> Mg2+ aq + CO32- aq
Mg2+ + SO42- -> MgSO4
- Magnesia binder:
Magnesia binder also contains magnesium compounds which can form hygroscopic salts. These are a source of magnesium which, in combination with sulfate ions, can lead to the formation of magnesium sulfate.
- Cement:
Cement also contains magnesium. To prevent damage, DIN 1164 stipulates that cement must not contain more than 5M% as magnesium can also be dissolved from cement and form sulfates. A further source of magnesium ions in solution can be road gritting salts as these often contain small amounts of magnesium chloride. Ions dissolved in the ground can also be problematic if transported to the surfaces by capillary action.
Solubility behavior[edit]
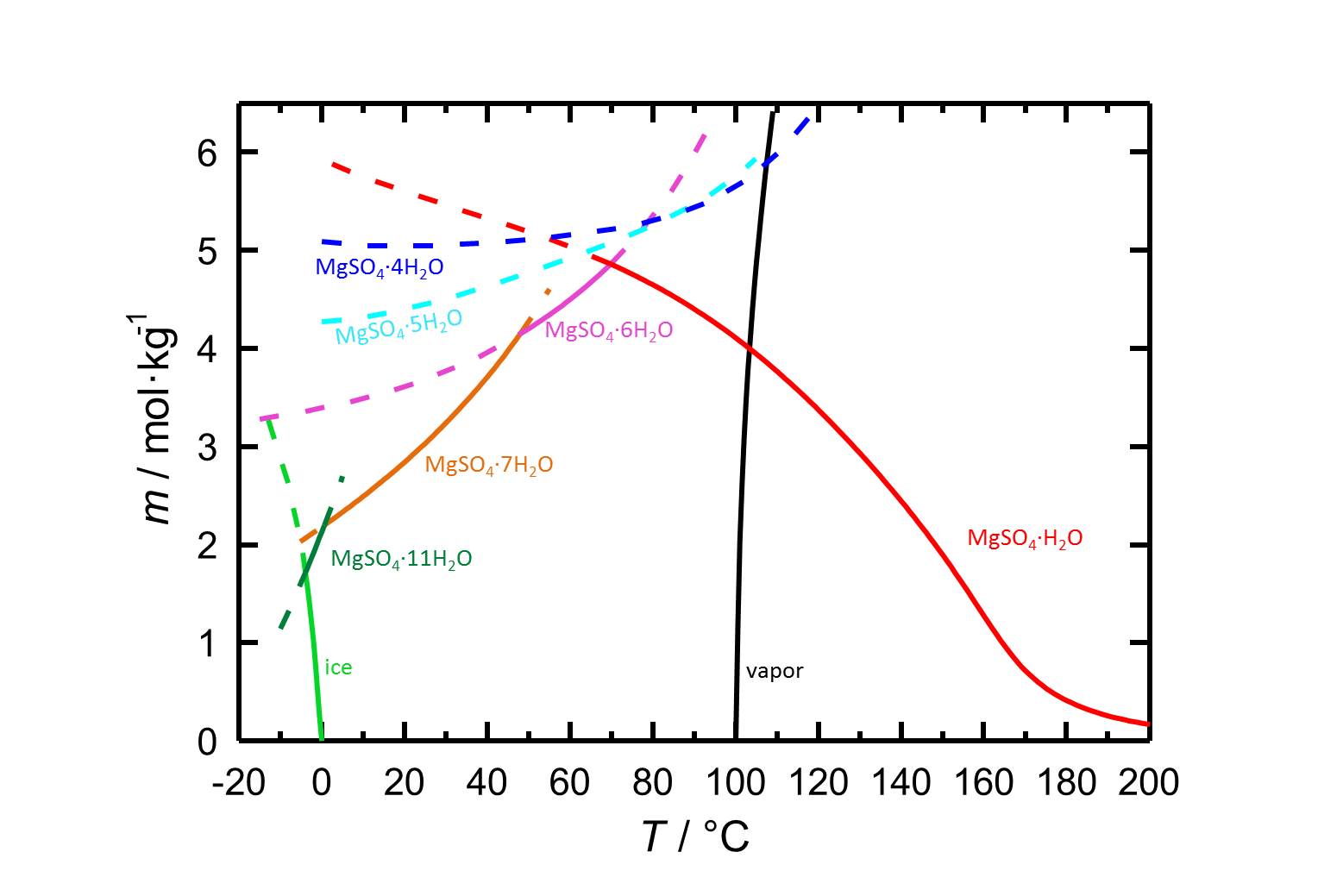
Author: Steiger, Michael; Linnow, Kirsten; Ehrhardt, Dorothee; Rohde, Mandy
 .
.
The water solubility of kieserite is 4.108 mol/kg at a temperature of 100 °C [Steiger.etal:2011a]Title: Decomposition reactions of magnesium sulfate hydrates and phase equilibria in the MgSO4-H2O and Na+-Mg2+-Cl--SO42--H2O systems with implications for Mars
Author: Steiger, Michael; Linnow, Kirsten; Ehrhardt, Dorothee; Rohde, Mandy . It subsequently belongs, like all discussed forms of magnesium sulphate with a solubility of clearly above 100 g/l (at 20 °C) to the group of very water soluble salts. This entails a high risk of salt mobility and frequent re-deposition of salts within the material matrix. Due to the influence of temperature on solubility, a rapid drop in temperature can result in the precipitation of salts [Mainusch:2001]Title: Erstellung einer Materialsammlung zur qualitativen Bestimmung bauschädlicher Salze für Fachleute der Restaurierung
. It subsequently belongs, like all discussed forms of magnesium sulphate with a solubility of clearly above 100 g/l (at 20 °C) to the group of very water soluble salts. This entails a high risk of salt mobility and frequent re-deposition of salts within the material matrix. Due to the influence of temperature on solubility, a rapid drop in temperature can result in the precipitation of salts [Mainusch:2001]Title: Erstellung einer Materialsammlung zur qualitativen Bestimmung bauschädlicher Salze für Fachleute der Restaurierung
Author: Mainusch, Nils
Hygroscopicity[edit]
The low hygroscopicity of the pure kieserite salt (illustrated by its 42% equilibrium RH) cannot be examined on its own.
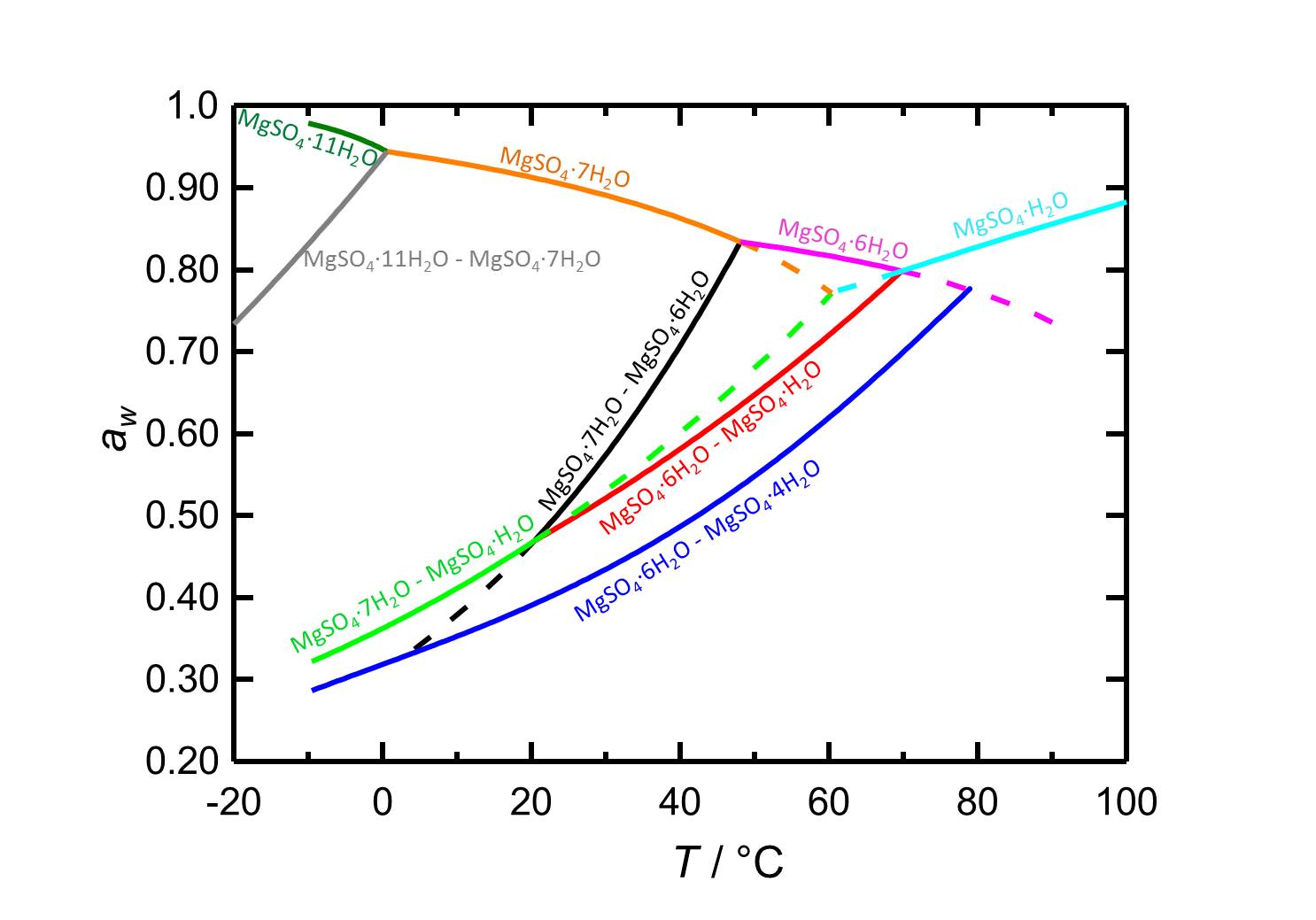
Author: Steiger, Michael; Linnow, Kirsten; Ehrhardt, Dorothee; Rohde, Mandy
 .
.
The sorption point of mixed systems, i.e. in the presence of other ions, is lower. The possibility of hygroscopic moisture absorption and associated problems is still present, in spite of the high deliquescence point [Mainusch:2001]Title: Erstellung einer Materialsammlung zur qualitativen Bestimmung bauschädlicher Salze für Fachleute der Restaurierung
Author: Mainusch, Nils
Hydration behavior[edit]
The six hydrate forms of magnesium sulfate listed above are shown to be stable compounds. With the exception of magnesium sulfate-12-hydrates, all the above crystal water phases of magnesium sulfate have been found on monuments. These predominantly occur as epsomite, hexahydrite, pentahydrite und kieserite.
Kieserite is the magnesium sulfate monohydrate. It can be formed through the dehydration of magnesium sulfate hydrates. A water-loss resultant reduction in volume occurs during this change. Increased relative humidity also results in an increased hydrate-water content within the magnesium sulfate. Kieserite is stable at room temperature (25°C) up to a RH of approx. 42 % , above this the change to hexahydrite or epsomite occurs. Hexahydrite is stable below 51 % RH, above this epsomite is formed. The phase changes can happen directly or via solution and re-crystallization. This results in the metastable existence of the lower hydration phase up to its deliquescence humidity. Above this RH the phase dissolves and a supercritical solution is formed, from which the hydrated phase crystallizes [Steiger.etal:2008]Title: Crystallization of sodium sulfate phases in porous materials: The phase diagram Na2SO4–H2O and the generation of stress
Author: Steiger, Michael; Asmussen, Sönke .
.
Transition reactions[edit]
Analytical validation[edit]
Micro chemistry[edit]
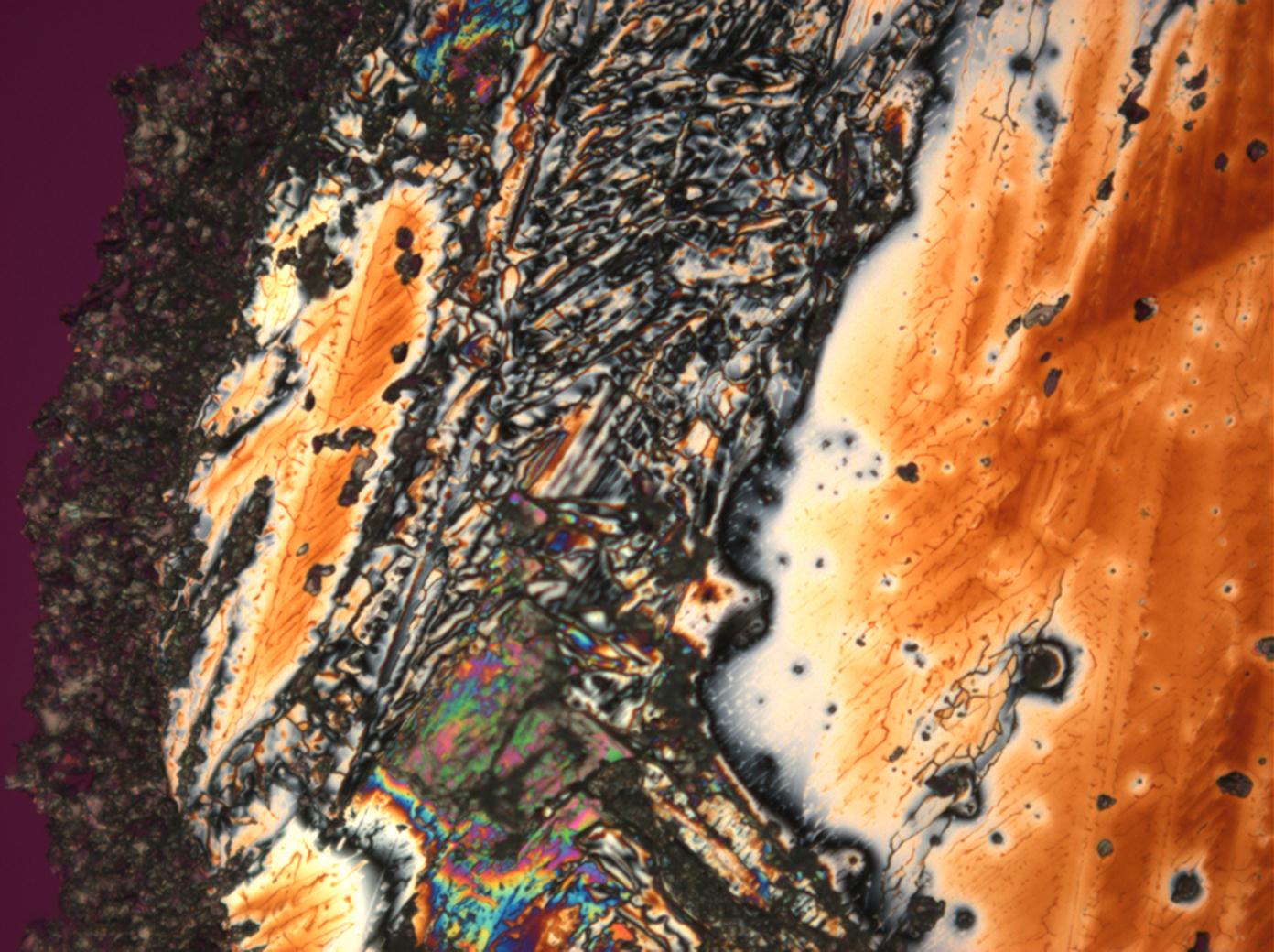
Microscopy[edit]
Polarizing microscope investigations:
During microscopic investigations, it is important to remember that hexahydrite is the stable phase in ambient room conditions, i.e. a temperature of approx. 20 °C and RH of approx. 40%. Subsequently, the environmental conditions need to be changed to allow the crystallization of kieserite to be observed.
Since it is an optically anisotropic mineral, it displays a birefringence when viewed through crossed polars. Its interference colors are low and within the first order. The refractive indices are around α=1,520 β=1,533 γ=1,584 with a birefringence of 0,01. In comparison with other sulfates and salts, the refractive indices are relatively high. The birefringence is in the lower region of the sulfates and very low in comparison to nitrates.
Weblinks
[edit]
- ↑ http://webmineral.com/data/Kieserite.shtml viewed on 29/07/2010
- ↑ http://www.mindat.org/min-2204.html viewed on 29/07/2010
Literature[edit]
| [Broul.etal:1981] | Elsevier (eds.) Broul M., Nyvlt J.; Soehnel O. (1981): Solubility in organic two component systems, Elsevier |  |
| [Dana:1951] | Dana E.S. (eds.) Dana J.D. (1951): Dana's System of Mineralogy, 7, Wiley & Sons |  |
| [Friedrich.etal:1961] | Friedrich K., Kuhn R., Strunz H. (1961): Identität von Wathlingenit mit Kieserit. In: Kali Steinsalz, 3 (7), 221-227 |  |
| [Mainusch:2001] | Mainusch, Nils (2001): Erstellung einer Materialsammlung zur qualitativen Bestimmung bauschädlicher Salze für Fachleute der Restaurierung, Diplomarbeit, HAWK Hochschule für angewandte Wissenschaft und Kunst Hildesheim/Holzminden/Göttingen, file:Diplomarbeit Nils Mainusch.pdf |   |
| [Steiger.etal:2008] | Steiger, Michael; Asmussen, Sönke (2008): Crystallization of sodium sulfate phases in porous materials: The phase diagram Na2SO4–H2O and the generation of stress. In: Geochimica et Cosmochimica Acta, 72 (17), 4291-4306, Url |  |
| [Steiger.etal:2011a] | Steiger, Michael; Linnow, Kirsten; Ehrhardt, Dorothee; Rohde, Mandy (2011): Decomposition reactions of magnesium sulfate hydrates and phase equilibria in the MgSO4-H2O and Na+-Mg2+-Cl--SO42--H2O systems with implications for Mars. In: Geochimica et Cosmochimica Act, 75 (12), 3600-3626, 10.1016/j.gca.2011.03.038, |  |